当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
5 V Stable Nitrile-Bearing Polymer Electrolyte with Aliphatic Segment as Internal Plasticizer
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-04-04 00:00:00 , DOI: 10.1021/acsaem.9b00103 Zhuo Li 1 , Yineng Zhao 1 , Wyatt E. Tenhaeff 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-04-04 00:00:00 , DOI: 10.1021/acsaem.9b00103 Zhuo Li 1 , Yineng Zhao 1 , Wyatt E. Tenhaeff 1
Affiliation
|
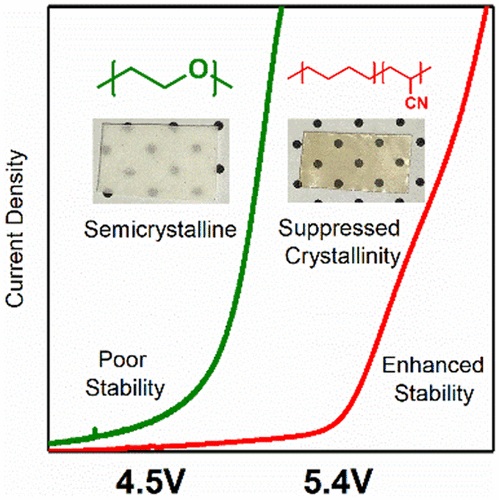
The electrochemical properties of polymer electrolytes based on poly(acrylonitrile-co-butadiene) and hydrogenated poly(acrylonitrile-co-butadiene) blended with lithium bis(trifluorosulfonyl)imide (LiTFSI) were characterized, showing that copolymerization of acrylonitrile with aliphatic comonomers is an effective means to develop conductive, yet oxidatively stable, polymer electrolytes. The copolymer compositions were both 40 wt % acrylonitrile, and the copolymer glass transition temperatures of NBR and HNBR were determined to be −17.7 and −24.3 °C, respectively. Differential scanning calorimetry (DSC) showed that the polymer electrolytes are amorphous when mixed with LiTFSI to a concentration of at least five nitrogen atoms for every one Li cation (N/Li = 5), and Fourier-transform infrared spectroscopy analysis of the nitrile stretch mode confirmed that the nitrile functional groups coordinate Li cations. At N/Li = 5, the ionic conductivity of HNBR:LiTFSI was determined to be 3.1 × 10–7 S/cm at 25 °C and 9.0 × 10–5 S/cm at 100 °C. In addition to dramatically enhancing the conductivity relative to polyacrylonitrile electrolytes, good oxidative stability was maintained. Linear scanning voltammetry showed that HNBR:LiTFSI was stable against oxidation to 5.4 V vs Li/Li+. Li–Li cell cycling and impedance evolution of the HNBR:LiTFSI–Li interface revealed that a substantial, yet stable, passivation layer is formed.
中文翻译:

具有脂肪链段作为内部增塑剂的5 V稳定的含腈聚合物电解质
基于聚(丙烯腈-共-丁二烯)和氢化聚(丙烯腈-共聚)的聚合物电解质的电化学性能与双(三氟磺酰基)酰亚胺锂(LiTFSI)混合的特征在于,表明丙烯腈与脂肪族共聚单体的共聚是开发导电但氧化稳定的聚合物电解质的有效手段。共聚物组合物均为40wt%的丙烯腈,并且确定NBR和HNBR的共聚物玻璃化转变温度分别为-17.7℃和-24.3℃。差示扫描量热法(DSC)表明,当与LiTFSI混合到每一个Li阳离子(N / Li = 5)的浓度至少为五个氮原子时,聚合物电解质是无定形的,并且腈拉伸的傅里叶变换红外光谱分析模式证实腈官能团配位锂阳离子。在N / Li = 5时,确定HNBR:LiTFSI的离子电导率为3。在25°C时为–7 S / cm,在100°C时为9.0×10 –5 S / cm。除了显着提高相对于聚丙烯腈电解质的电导率外,还保持了良好的氧化稳定性。线性扫描伏安法表明,相对于Li / Li +,HNBR:LiTFSI的抗氧化稳定性达到5.4V 。Li-Li电池循环和HNBR:LiTFSI-Li界面的阻抗演变表明,形成了坚固但稳定的钝化层。
更新日期:2019-04-04
中文翻译:

具有脂肪链段作为内部增塑剂的5 V稳定的含腈聚合物电解质
基于聚(丙烯腈-共-丁二烯)和氢化聚(丙烯腈-共聚)的聚合物电解质的电化学性能与双(三氟磺酰基)酰亚胺锂(LiTFSI)混合的特征在于,表明丙烯腈与脂肪族共聚单体的共聚是开发导电但氧化稳定的聚合物电解质的有效手段。共聚物组合物均为40wt%的丙烯腈,并且确定NBR和HNBR的共聚物玻璃化转变温度分别为-17.7℃和-24.3℃。差示扫描量热法(DSC)表明,当与LiTFSI混合到每一个Li阳离子(N / Li = 5)的浓度至少为五个氮原子时,聚合物电解质是无定形的,并且腈拉伸的傅里叶变换红外光谱分析模式证实腈官能团配位锂阳离子。在N / Li = 5时,确定HNBR:LiTFSI的离子电导率为3。在25°C时为–7 S / cm,在100°C时为9.0×10 –5 S / cm。除了显着提高相对于聚丙烯腈电解质的电导率外,还保持了良好的氧化稳定性。线性扫描伏安法表明,相对于Li / Li +,HNBR:LiTFSI的抗氧化稳定性达到5.4V 。Li-Li电池循环和HNBR:LiTFSI-Li界面的阻抗演变表明,形成了坚固但稳定的钝化层。

































 京公网安备 11010802027423号
京公网安备 11010802027423号