当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tip-Enhanced Infrared Difference-Nanospectroscopy of the Proton Pump Activity of Bacteriorhodopsin in Single Purple Membrane Patches
Nano Letters ( IF 9.6 ) Pub Date : 2019-04-05 00:00:00 , DOI: 10.1021/acs.nanolett.9b00512 Valeria Giliberti 1 , Raffaella Polito 2 , Eglof Ritter 3 , Matthias Broser 3 , Peter Hegemann 3 , Ljiljana Puskar 4 , Ulrich Schade 4 , Laura Zanetti-Polzi 5 , Isabella Daidone 5 , Stefano Corni 6, 7 , Francesco Rusconi 8 , Paolo Biagioni 8 , Leonetta Baldassarre 2 , Michele Ortolani 1, 2
Nano Letters ( IF 9.6 ) Pub Date : 2019-04-05 00:00:00 , DOI: 10.1021/acs.nanolett.9b00512 Valeria Giliberti 1 , Raffaella Polito 2 , Eglof Ritter 3 , Matthias Broser 3 , Peter Hegemann 3 , Ljiljana Puskar 4 , Ulrich Schade 4 , Laura Zanetti-Polzi 5 , Isabella Daidone 5 , Stefano Corni 6, 7 , Francesco Rusconi 8 , Paolo Biagioni 8 , Leonetta Baldassarre 2 , Michele Ortolani 1, 2
Affiliation
|
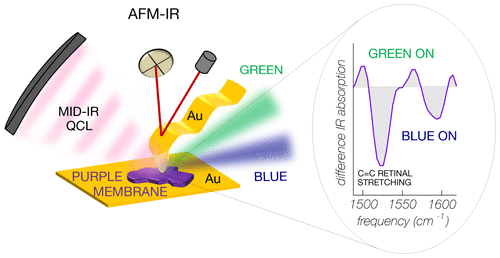
Photosensitive proteins embedded in the cell membrane (about 5 nm thickness) act as photoactivated proton pumps, ion gates, enzymes, or more generally, as initiators of stimuli for the cell activity. They are composed of a protein backbone and a covalently bound cofactor (e.g. the retinal chromophore in bacteriorhodopsin (BR), channelrhodopsin, and other opsins). The light-induced conformational changes of both the cofactor and the protein are at the basis of the physiological functions of photosensitive proteins. Despite the dramatic development of microscopy techniques, investigating conformational changes of proteins at the membrane monolayer level is still a big challenge. Techniques based on atomic force microscopy (AFM) can detect electric currents through protein monolayers and even molecular binding forces in single-protein molecules but not the conformational changes. For the latter, Fourier-transform infrared spectroscopy (FTIR) using difference-spectroscopy mode is typically employed, but it is performed on macroscopic liquid suspensions or thick films containing large amounts of purified photosensitive proteins. In this work, we develop AFM-assisted, tip-enhanced infrared difference-nanospectroscopy to investigate light-induced conformational changes of the bacteriorhodopsin mutant D96N in single submicrometric native purple membrane patches. We obtain a significant improvement compared with the signal-to-noise ratio of standard IR nanospectroscopy techniques by exploiting the field enhancement in the plasmonic nanogap that forms between a gold-coated AFM probe tip and an ultraflat gold surface, as further supported by electromagnetic and thermal simulations. IR difference-spectra in the 1450–1800 cm–1 range are recorded from individual patches as thin as 10 nm, with a diameter of less than 500 nm, well beyond the diffraction limit for FTIR microspectroscopy. We find clear spectroscopic evidence of a branching of the photocycle for BR molecules in direct contact with the gold surfaces, with equal amounts of proteins either following the standard proton-pump photocycle or being trapped in an intermediate state not directly contributing to light-induced proton transport. Our results are particularly relevant for BR-based optoelectronic and energy-harvesting devices, where BR molecular monolayers are put in contact with metal surfaces, and, more generally, for AFM-based IR spectroscopy studies of conformational changes of proteins embedded in intrinsically heterogeneous native cell membranes.
中文翻译:

紫色膜单个膜片中细菌视紫红质的质子泵活性的针尖-增强红外差光谱-纳米光谱
嵌入细胞膜中的光敏蛋白(约5 nm厚)充当光活化的质子泵,离子门,酶,或更一般地,充当细胞活动刺激的引发剂。它们由蛋白质主链和共价结合的辅因子(例如,细菌视紫红质(BR),视紫红质和其他视蛋白中的视网膜发色团)组成。光诱导的辅因子和蛋白质的构象变化是光敏蛋白质生理功能的基础。尽管显微技术得到了巨大的发展,但在膜单层水平研究蛋白质的构象变化仍然是一个很大的挑战。基于原子力显微镜(AFM)的技术可以检测通过蛋白质单层的电流,甚至可以检测单蛋白质分子中的分子结合力,但不能检测构象变化。对于后者,通常采用使用差分光谱模式的傅立叶变换红外光谱(FTIR),但它是在宏观液体悬浮液或含有大量纯化光敏蛋白的厚膜上进行的。在这项工作中,我们开发了AFM辅助的,尖端增强的红外差异纳米显微镜,以研究单个亚微米天然紫色膜片中细菌视紫红质突变体D96N的光诱导构象变化。与标准IR纳米光谱技术的信噪比相比,我们通过利用镀金AFM探针尖端和超平坦金表面之间形成的等离子纳米间隙的场增强获得了显着的改进,电磁场和电磁场进一步支持了这种现象。热模拟。红外差光谱在1450–1800 cm记录了单个贴片的–1范围,其厚度仅为10 nm,直径小于500 nm,远远超出了FTIR显微技术的衍射极限。我们发现清晰的光谱学证据表明,与金表面直接接触的BR分子具有光周期的分支,其中等量的蛋白质要么遵循标准的质子泵光周期,要么被捕获在中间状态而不会直接导致光诱导的质子运输。我们的结果与基于BR的光电和能量收集设备特别相关,在这些设备中,BR分子单分子层与金属表面接触,更普遍地,对于基于AFM的IR光谱研究,固有异质天然分子中嵌入的蛋白质的构象变化细胞膜。
更新日期:2019-04-05
中文翻译:

紫色膜单个膜片中细菌视紫红质的质子泵活性的针尖-增强红外差光谱-纳米光谱
嵌入细胞膜中的光敏蛋白(约5 nm厚)充当光活化的质子泵,离子门,酶,或更一般地,充当细胞活动刺激的引发剂。它们由蛋白质主链和共价结合的辅因子(例如,细菌视紫红质(BR),视紫红质和其他视蛋白中的视网膜发色团)组成。光诱导的辅因子和蛋白质的构象变化是光敏蛋白质生理功能的基础。尽管显微技术得到了巨大的发展,但在膜单层水平研究蛋白质的构象变化仍然是一个很大的挑战。基于原子力显微镜(AFM)的技术可以检测通过蛋白质单层的电流,甚至可以检测单蛋白质分子中的分子结合力,但不能检测构象变化。对于后者,通常采用使用差分光谱模式的傅立叶变换红外光谱(FTIR),但它是在宏观液体悬浮液或含有大量纯化光敏蛋白的厚膜上进行的。在这项工作中,我们开发了AFM辅助的,尖端增强的红外差异纳米显微镜,以研究单个亚微米天然紫色膜片中细菌视紫红质突变体D96N的光诱导构象变化。与标准IR纳米光谱技术的信噪比相比,我们通过利用镀金AFM探针尖端和超平坦金表面之间形成的等离子纳米间隙的场增强获得了显着的改进,电磁场和电磁场进一步支持了这种现象。热模拟。红外差光谱在1450–1800 cm记录了单个贴片的–1范围,其厚度仅为10 nm,直径小于500 nm,远远超出了FTIR显微技术的衍射极限。我们发现清晰的光谱学证据表明,与金表面直接接触的BR分子具有光周期的分支,其中等量的蛋白质要么遵循标准的质子泵光周期,要么被捕获在中间状态而不会直接导致光诱导的质子运输。我们的结果与基于BR的光电和能量收集设备特别相关,在这些设备中,BR分子单分子层与金属表面接触,更普遍地,对于基于AFM的IR光谱研究,固有异质天然分子中嵌入的蛋白质的构象变化细胞膜。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号