当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atmospheric oxidation reactions of imidazole initiated by hydroxyl radicals: kinetics and mechanism of reactions and atmospheric implications†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2019-04-05 00:00:00 , DOI: 10.1039/c9cp00632j Zahra Safaei 1, 2, 3, 4, 5 , Abolfazl Shiroudi 6, 7, 8, 9, 10 , Ehsan Zahedi 8, 10, 11, 12, 13 , Mika Sillanpää 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2019-04-05 00:00:00 , DOI: 10.1039/c9cp00632j Zahra Safaei 1, 2, 3, 4, 5 , Abolfazl Shiroudi 6, 7, 8, 9, 10 , Ehsan Zahedi 8, 10, 11, 12, 13 , Mika Sillanpää 1, 2, 3, 4, 5
Affiliation
|
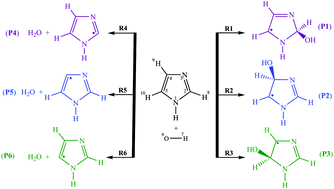
The atmospheric oxidation mechanism of imidazole initiated by hydroxyl radicals is investigated via OH-addition and H-abstraction pathways by quantum chemistry calculations at the M06-2X/aug-cc-pVTZ level of theory coupled with reaction kinetics calculations using statistical Rice–Ramsperger–Kassel–Marcus (RRKM) theory and transition state theory (TST). It was found that OH addition proceeds more rapidly than H-abstraction by several orders of magnitude. Moreover, H-abstraction reactions with submerged barriers exhibit positive temperature dependence. Effects of reaction temperature and pressure on the reaction between imidazole and OH radicals are studied by means of RRKM calculations. Effective rate coefficients involve two-step mechanisms. According to the experiment, the obtained branching ratios show that the kinetically most efficient process corresponds to OH addition onto a carbon atom which is adjacent to a nitrogen atom having a lower energy barrier. These ratios also reveal that the regioselectivity of the oxidation reaction decreases with increasing temperatures and decreasing pressures. Because of negative activation energies, pressures larger than 100 bar are required to reach the high pressure limit. The atmospheric lifetime of imidazole in the presence of OH radicals is estimated to be ∼4.74 days, based on the calculated overall kinetic rate constant of 1.22 × 10−12 cm3 molecule−1 s−1 at a pressure of 1 bar and nearly ambient temperature. NBO analysis demonstrates that the calculated energy barriers are dictated by charge transfer effects and aromaticity changes because of the delocalization of nitrogen lone pairs to empty π* orbitals.
中文翻译:

羟自由基引发的咪唑在大气中的氧化反应:动力学和反应机理以及对大气的影响†
由羟基自由基引发咪唑的大气氧化机理进行了研究通过在M06-2X / aug-cc-pVTZ理论水平上通过量子化学计算进行的OH加成和H吸收途径,以及使用莱斯-拉姆斯伯格-卡塞尔-马库斯(RRKM)统计理论和过渡态理论(TST)进行的反应动力学计算)。已经发现,OH的添加比H-的吸收更快地进行了几个数量级。此外,具有浸没式阻挡层的H吸收反应表现出正温度依赖性。通过RRKM计算研究了反应温度和压力对咪唑与OH自由基之间反应的影响。有效的利率系数涉及两步机制。根据实验,所获得的支化比表明,动力学上最有效的过程相当于将OH加成到与具有较低能垒的氮原子相邻的碳原子上。这些比率还表明,氧化反应的区域选择性随着温度和压力的降低而降低。由于负激活能,需要超过100 bar的压力才能达到高压极限。基于计算的总动力学速率常数1.22×10,估计在存在OH自由基的情况下咪唑的大气寿命约为4.74天。要达到高压极限,必须使用大于100 bar的压力。基于计算的总动力学速率常数1.22×10,估计在存在OH自由基的情况下咪唑的大气寿命约为4.74天。要达到高压极限,需要大于100 bar的压力。基于计算的总动力学速率常数1.22×10,估计在存在OH自由基的情况下咪唑的大气寿命约为4.74天。-12 cm 3分子-1 s -1在1 bar的压力下和接近环境温度。NBO分析表明,所计算出的能垒是由电荷转移效应和芳香性变化所决定的,这是由于氮孤对成离域为空的π*轨道所致。
更新日期:2019-04-05
中文翻译:

羟自由基引发的咪唑在大气中的氧化反应:动力学和反应机理以及对大气的影响†
由羟基自由基引发咪唑的大气氧化机理进行了研究通过在M06-2X / aug-cc-pVTZ理论水平上通过量子化学计算进行的OH加成和H吸收途径,以及使用莱斯-拉姆斯伯格-卡塞尔-马库斯(RRKM)统计理论和过渡态理论(TST)进行的反应动力学计算)。已经发现,OH的添加比H-的吸收更快地进行了几个数量级。此外,具有浸没式阻挡层的H吸收反应表现出正温度依赖性。通过RRKM计算研究了反应温度和压力对咪唑与OH自由基之间反应的影响。有效的利率系数涉及两步机制。根据实验,所获得的支化比表明,动力学上最有效的过程相当于将OH加成到与具有较低能垒的氮原子相邻的碳原子上。这些比率还表明,氧化反应的区域选择性随着温度和压力的降低而降低。由于负激活能,需要超过100 bar的压力才能达到高压极限。基于计算的总动力学速率常数1.22×10,估计在存在OH自由基的情况下咪唑的大气寿命约为4.74天。要达到高压极限,必须使用大于100 bar的压力。基于计算的总动力学速率常数1.22×10,估计在存在OH自由基的情况下咪唑的大气寿命约为4.74天。要达到高压极限,需要大于100 bar的压力。基于计算的总动力学速率常数1.22×10,估计在存在OH自由基的情况下咪唑的大气寿命约为4.74天。-12 cm 3分子-1 s -1在1 bar的压力下和接近环境温度。NBO分析表明,所计算出的能垒是由电荷转移效应和芳香性变化所决定的,这是由于氮孤对成离域为空的π*轨道所致。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号