当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deamidation of Protonated Asparagine-Valine Investigated by a Combined Spectroscopic, Guided Ion Beam, and Theoretical Study.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-27 , DOI: 10.1021/acs.jpca.7b12348 L J M Kempkes 1 , G C Boles 2 , J Martens 1 , G Berden 1 , P B Armentrout 2 , J Oomens 1, 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-02-27 , DOI: 10.1021/acs.jpca.7b12348 L J M Kempkes 1 , G C Boles 2 , J Martens 1 , G Berden 1 , P B Armentrout 2 , J Oomens 1, 3
Affiliation
|
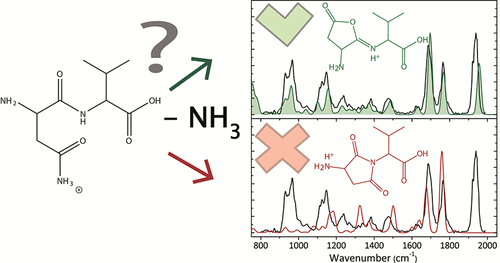
Peptide deamidation of asparaginyl residues is a spontaneous post-translational modification that is believed to play a role in aging and several diseases. It is also a well-known small-molecule loss channel in the MS/MS spectra of protonated peptides. Here we investigate the deamidation reaction, as well as other decomposition pathways, of the protonated dipeptide asparagine-valine ([AsnVal + H]+) upon low-energy activation in a mass spectrometer. Using a combination of infrared ion spectroscopy, guided ion beam tandem mass spectrometry, and theoretical calculations, we have been able to identify product ion structures and determine the energetics and mechanisms for decomposition. Deamidation proceeds via ammonia loss from the asparagine side chain, initiated by a nucleophilic attack of the peptide bond oxygen on the γ-carbon of the Asn side chain. This leads to the formation of a furanone ring containing product ion characterized by a threshold energy of 129 ± 5 kJ/mol (15 kJ/mol higher in energy than dehydration of [AsnVal + H]+, the lowest energy dissociation channel available to the system). Competing formation of a succinimide ring containing product, as has been observed for protonated asparagine-glycine ([AsnGly + H]+) and asparagine-alanine ([AsnAla + H]+), was not observed here. Quantum-chemical modeling of the reaction pathways confirms these subtle differences in dissociation behavior. Measured reaction thresholds are in agreement with predicted theoretical reaction energies computed at several levels of theory.
中文翻译:

通过结合光谱、引导离子束和理论研究研究质子化天冬酰胺-缬氨酸的脱酰胺化。
天冬酰胺残基的肽脱酰胺是一种自发的翻译后修饰,被认为在衰老和多种疾病中发挥作用。它也是质子化肽 MS/MS 谱图中众所周知的小分子损失通道。在这里,我们研究了质子化二肽天冬酰胺-缬氨酸 ([AsnVal + H]+) 在质谱仪中低能激活时的脱酰胺反应以及其他分解途径。通过结合红外离子光谱、引导离子束串联质谱和理论计算,我们已经能够识别产物离子结构并确定分解的能量和机制。脱酰胺作用是通过天冬酰胺侧链的氨损失进行的,这是由天冬酰胺侧链的 γ-碳上的肽键氧的亲核攻击引发的。这导致形成含有产物离子的呋喃酮环,其阈值能量为 129 ± 5 kJ/mol(比 [AsnVal + H]+ 的脱水能量高 15 kJ/mol,这是可利用的最低能量解离通道)系统)。正如在质子化天冬酰胺-甘氨酸 ([AsnGly + H]+) 和天冬酰胺-丙氨酸 ([AsnAla + H]+) 中观察到的那样,此处没有观察到含有琥珀酰亚胺环的产物的竞争形成。反应途径的量子化学模型证实了解离行为的这些细微差异。测量的反应阈值与在多个理论水平上计算的预测理论反应能量一致。
更新日期:2018-02-13
中文翻译:

通过结合光谱、引导离子束和理论研究研究质子化天冬酰胺-缬氨酸的脱酰胺化。
天冬酰胺残基的肽脱酰胺是一种自发的翻译后修饰,被认为在衰老和多种疾病中发挥作用。它也是质子化肽 MS/MS 谱图中众所周知的小分子损失通道。在这里,我们研究了质子化二肽天冬酰胺-缬氨酸 ([AsnVal + H]+) 在质谱仪中低能激活时的脱酰胺反应以及其他分解途径。通过结合红外离子光谱、引导离子束串联质谱和理论计算,我们已经能够识别产物离子结构并确定分解的能量和机制。脱酰胺作用是通过天冬酰胺侧链的氨损失进行的,这是由天冬酰胺侧链的 γ-碳上的肽键氧的亲核攻击引发的。这导致形成含有产物离子的呋喃酮环,其阈值能量为 129 ± 5 kJ/mol(比 [AsnVal + H]+ 的脱水能量高 15 kJ/mol,这是可利用的最低能量解离通道)系统)。正如在质子化天冬酰胺-甘氨酸 ([AsnGly + H]+) 和天冬酰胺-丙氨酸 ([AsnAla + H]+) 中观察到的那样,此处没有观察到含有琥珀酰亚胺环的产物的竞争形成。反应途径的量子化学模型证实了解离行为的这些细微差异。测量的反应阈值与在多个理论水平上计算的预测理论反应能量一致。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号