Nature Communications ( IF 14.7 ) Pub Date : 2019-04-04 , DOI: 10.1038/s41467-019-09392-7 Tao Cheng , Dong Xue Shen , Miao Meng , Suman Mallick , Lijiu Cao , Nathan J. Patmore , Hong Li Zhang , Shan Feng Zou , Huo Wen Chen , Yi Qin , Yi Yang Wu , Chun Y. Liu
|
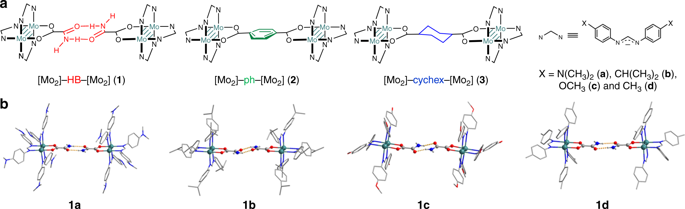
Thermal electron transfer through hydrogen bonds remains largely unexplored. Here we report the study of electron transfer through amide-amide hydrogen bonded interfaces in mixed-valence complexes with covalently bonded Mo2 units as the electron donor and acceptor. The rate constants for electron transfer through the dual hydrogen bonds across a distance of 12.5 Å are on the order of ∼ 1010 s−1, as determined by optical analysis based on Marcus–Hush theory and simulation of ν(NH) vibrational band broadening, with the electron transfer efficiencies comparable to that of π conjugated bridges. This work demonstrates that electron transfer across a hydrogen bond may proceed via the known proton-coupled pathway, as well as an overlooked proton-uncoupled pathway that does not involve proton transfer. A mechanistic switch between the two pathways can be achieved by manipulation of the strengths of electronic coupling and hydrogen bonding. The knowledge of the non-proton coupled pathway has shed light on charge and energy transport in biological systems.
中文翻译:

通过质子偶联和非偶联途径在氢键界面上进行有效的电子转移
通过氢键的热电子转移在很大程度上仍未被探索。在这里,我们报告了通过价键Mo 2单元作为电子供体和受体的混合价配合物中的酰胺-氢键结合界面电子转移的研究。电子通过双氢键跨12.5Å的传输速率常数约为10 10 s -1由基于Marcus-Hush理论的光学分析和ν(NH)振动带展宽的模拟确定,其电子传输效率可与π共轭电桥相媲美。这项工作表明,跨氢键的电子转移可能会通过已知的质子偶联途径进行,也可能会通过不涉及质子转移的被忽略的质子解偶联途径进行。可以通过控制电子耦合和氢键的强度来实现两条途径之间的机械转换。非质子耦合途径的知识揭示了生物系统中的电荷和能量传输。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号