当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unexpected Dissociation Mechanism of Sodiated N-Acetylglucosamine and N-Acetylgalactosamine
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-04-04 00:00:00 , DOI: 10.1021/acs.jpca.9b00934
Cheng-chau Chiu,Shang-Ting Tsai,Po-Jen Hsu,Hai Thi Huynh,Jien-Lian Chen,Huu Trong Phan,Shih-Pei Huang,Hou-Yu Lin,Jer-Lai Kuo,Chi-Kung Ni
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-04-04 00:00:00 , DOI: 10.1021/acs.jpca.9b00934
Cheng-chau Chiu,Shang-Ting Tsai,Po-Jen Hsu,Hai Thi Huynh,Jien-Lian Chen,Huu Trong Phan,Shih-Pei Huang,Hou-Yu Lin,Jer-Lai Kuo,Chi-Kung Ni
|
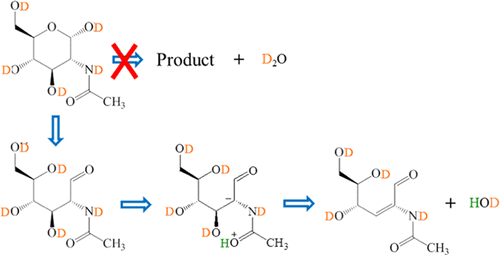
The mechanism for the collision-induced dissociation (CID) of two sodiated N-acetylhexosamines (HexNAc), N-acetylglucosamine (GlcNAc), and N-acetylgalactosamine (GalNAc), was studied using quantum-chemistry calculations and resonance excitation in a low-pressure linear ion trap. Experimental results show that the major dissociation channel of the isotope labeled [1-18O, D5]-HexNAc is the dehydration by eliminating HDO, where OD comes from the OD group at C3. Dissociation channels of minor importance include the 0,2A cross-ring dissociation. No difference has been observed between the CID spectra of the α- and β-anomers of the same HexNAc. At variance, the CID spectra of GlcNAc and GalNAc showed some differences, which can be used to distinguish the two structures. It was observed in CID experiments involving disaccharides with a HexNAc at the nonreducing end that a β-HexNAc shows a larger dissociation branching ratio for the glycosidic bond cleavage than the α-anomer. This finding can be exploited for the rapid identification of the anomeric configuration at the glycosidic bond of HexNAc-R′ (R′ = sugar) structures. The experimental observations indicating that the dissociation mechanisms of HexNAcs are significantly different from those of hexoses were explained by quantum-chemistry calculations. Calculations show that ring opening is the major channel for HexNAcs in a ring form. After ring opening, dehydration shows the lowest barrier. In contrast, the glycosidic bond cleavage becomes the major channel for HexNAcs at the nonreducing end of a disaccharide. This reaction has a lower barrier for β-HexNAcs as compared with the barrier of the corresponding α-anomers, consistent with the higher branching ratio for β-HexNAcs observed in experiment.
中文翻译:

N-乙酰氨基葡萄糖和N-乙酰半乳糖胺的意外离解机理
为二的碰撞诱导解离(CID)的机制sodiated Ñ -acetylhexosamines(HexNAc),Ñ乙酰氨基葡萄糖(GlcNAc的),和Ñ -acetylgalactosamine胺(GalNAc),使用量子化学计算和共振激发在低了研究压力线性离子阱。实验结果表明,标记为[1- 18 O,D 5 ] -HexNAc的同位素的主要解离通道是通过消除HDO进行的脱水,其中OD来自C3的OD组。无关紧要的解离渠道包括0,2交叉环解离。在同一HexNAc的α-和β-异头物的CID光谱之间未观察到差异。GlcNAc和GalNAc的CID谱有一定差异,可以用来区分这两种结构。在涉及在非还原端带有HexNAc的二糖的CID实验中观察到,β-HexNAc对糖苷键的裂解显示出比α-端基异构体更大的解离支化比。该发现可用于快速鉴定HexNAc-R'(R'=糖)结构的糖苷键处的异头构型。实验观察表明,HexNAcs的解离机理与己糖的解离机理明显不同,这是通过量子化学计算来解释的。计算表明,开环是环形HexNAcs的主要通道。开环后,脱水显示出最低的阻隔性。相反,在二糖的非还原端,糖苷键的切割成为HexNAcs的主要通道。与相应的α-端基异构体的势垒相比,该反应对β-HexNAcs的势垒较低,这与在实验中观察到的对β-HexNAcs的较高支化率相一致。
更新日期:2019-04-04
中文翻译:

N-乙酰氨基葡萄糖和N-乙酰半乳糖胺的意外离解机理
为二的碰撞诱导解离(CID)的机制sodiated Ñ -acetylhexosamines(HexNAc),Ñ乙酰氨基葡萄糖(GlcNAc的),和Ñ -acetylgalactosamine胺(GalNAc),使用量子化学计算和共振激发在低了研究压力线性离子阱。实验结果表明,标记为[1- 18 O,D 5 ] -HexNAc的同位素的主要解离通道是通过消除HDO进行的脱水,其中OD来自C3的OD组。无关紧要的解离渠道包括0,2交叉环解离。在同一HexNAc的α-和β-异头物的CID光谱之间未观察到差异。GlcNAc和GalNAc的CID谱有一定差异,可以用来区分这两种结构。在涉及在非还原端带有HexNAc的二糖的CID实验中观察到,β-HexNAc对糖苷键的裂解显示出比α-端基异构体更大的解离支化比。该发现可用于快速鉴定HexNAc-R'(R'=糖)结构的糖苷键处的异头构型。实验观察表明,HexNAcs的解离机理与己糖的解离机理明显不同,这是通过量子化学计算来解释的。计算表明,开环是环形HexNAcs的主要通道。开环后,脱水显示出最低的阻隔性。相反,在二糖的非还原端,糖苷键的切割成为HexNAcs的主要通道。与相应的α-端基异构体的势垒相比,该反应对β-HexNAcs的势垒较低,这与在实验中观察到的对β-HexNAcs的较高支化率相一致。

































 京公网安备 11010802027423号
京公网安备 11010802027423号