当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanoenabled Modulation of Acidic Tumor Microenvironment Reverses Anergy of Infiltrating T Cells and Potentiates Anti-PD-1 Therapy
Nano Letters ( IF 9.6 ) Pub Date : 2019-04-03 00:00:00 , DOI: 10.1021/acs.nanolett.8b04296 Yu-Xue Zhang 1 , Yang-Yang Zhao 1 , Jizhou Shen 1 , Xun Sun 1 , Yi Liu 1 , Hang Liu 1 , Yucai Wang 1 , Jun Wang 2
Nano Letters ( IF 9.6 ) Pub Date : 2019-04-03 00:00:00 , DOI: 10.1021/acs.nanolett.8b04296 Yu-Xue Zhang 1 , Yang-Yang Zhao 1 , Jizhou Shen 1 , Xun Sun 1 , Yi Liu 1 , Hang Liu 1 , Yucai Wang 1 , Jun Wang 2
Affiliation
|
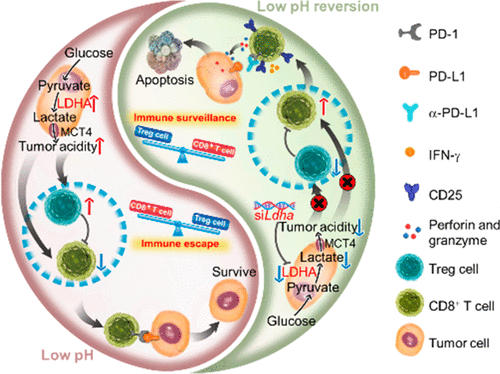
While tumor-infiltrating cytotoxic T lymphocytes play a critical role in controlling tumor development, they are generally impotent in an acidic tumor microenvironment. Systemic treatment to neutralize tumor acidity thus holds promise for the reversal of the anergic state of T cells and the improvement of T cell-associated immunotherapy. Herein, we report a proof-of-concept of RNAi nanoparticle-mediated therapeutic reversion of tumor acidity to restore the antitumor functions of T cells and potentiate the checkpoint blockade therapy. Our strategy utilized an in vivo optimized vesicular cationic lipid-assisted nanoparticle, as opposed to its micellar counterpart, to mediate systematic knockdown of lactate dehydrogenase A (LDHA) in tumor cells. The treatment resulted in the reprogramming of pyruvate metabolism, a reduction of the production of lactate, and the neutralization of the tumor pH. In immunocompetent syngeneic melanoma and breast tumor models, neutralization of tumor acidity increased infiltration with CD8+ T and NK cells, decreased the number of immunosuppressive T cells, and thus significantly inhibited the growth of tumors. Furthermore, the restoration of tumoral pH potentiated checkpoint inhibition therapy using the antibody of programmed cell death protein 1 (PD-1). However, in immunodeficient B6/Rag1–/– and NOG mice, the same treatment failed to control tumor growth, further proving that the attenuation of tumor growth by tumor acidity modulation was attributable to the activation of tumor-infiltrating immune cells.
中文翻译:

酸性肿瘤微环境的纳米调制逆转了浸润T细胞的无能,并增强了抗PD-1治疗的能力。
虽然浸润肿瘤的细胞毒性T淋巴细胞在控制肿瘤发展中起着关键作用,但它们通常在酸性肿瘤微环境中无能为力。因此,中和肿瘤酸度的全身治疗有望逆转T细胞的无反应状态,并改善T细胞相关的免疫疗法。在本文中,我们报告了RNAi纳米粒子介导的治疗性酸性还原的概念证明,以恢复T细胞的抗肿瘤功能并增强检查点封锁疗法。我们的策略是利用体内优化的囊泡阳离子脂质辅助纳米颗粒,而不是其胶束对应物,来介导肿瘤细胞中乳酸脱氢酶A(LDHA)的系统性敲低。治疗导致丙酮酸代谢的重新编程,减少乳酸的产生,并中和肿瘤的pH。在具有免疫功能的同基因黑色素瘤和乳腺肿瘤模型中,肿瘤酸性的中和会增加CD8的浸润+ T和NK细胞减少了免疫抑制性T细胞的数量,从而显着抑制了肿瘤的生长。此外,使用程序性细胞死亡蛋白1(PD-1)抗体恢复肿瘤pH增强的检查点抑制疗法。但是,在免疫缺陷的B6 / Rag1 -/-和NOG小鼠中,相同的治疗方法无法控制肿瘤的生长,进一步证明了通过肿瘤酸度调节来减弱肿瘤的生长归因于肿瘤浸润性免疫细胞的激活。
更新日期:2019-04-03
中文翻译:

酸性肿瘤微环境的纳米调制逆转了浸润T细胞的无能,并增强了抗PD-1治疗的能力。
虽然浸润肿瘤的细胞毒性T淋巴细胞在控制肿瘤发展中起着关键作用,但它们通常在酸性肿瘤微环境中无能为力。因此,中和肿瘤酸度的全身治疗有望逆转T细胞的无反应状态,并改善T细胞相关的免疫疗法。在本文中,我们报告了RNAi纳米粒子介导的治疗性酸性还原的概念证明,以恢复T细胞的抗肿瘤功能并增强检查点封锁疗法。我们的策略是利用体内优化的囊泡阳离子脂质辅助纳米颗粒,而不是其胶束对应物,来介导肿瘤细胞中乳酸脱氢酶A(LDHA)的系统性敲低。治疗导致丙酮酸代谢的重新编程,减少乳酸的产生,并中和肿瘤的pH。在具有免疫功能的同基因黑色素瘤和乳腺肿瘤模型中,肿瘤酸性的中和会增加CD8的浸润+ T和NK细胞减少了免疫抑制性T细胞的数量,从而显着抑制了肿瘤的生长。此外,使用程序性细胞死亡蛋白1(PD-1)抗体恢复肿瘤pH增强的检查点抑制疗法。但是,在免疫缺陷的B6 / Rag1 -/-和NOG小鼠中,相同的治疗方法无法控制肿瘤的生长,进一步证明了通过肿瘤酸度调节来减弱肿瘤的生长归因于肿瘤浸润性免疫细胞的激活。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号