当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
VPS33B interacts with NESG1 to modulate EGFR/PI3K/AKT/c-Myc/P53/miR-133a-3p signaling and induce 5-fluorouracil sensitivity in nasopharyngeal carcinoma.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-04-03 , DOI: 10.1038/s41419-019-1457-9 Zixi Liang 1, 2 , Zhen Liu 2, 3 , Chao Cheng 2, 4 , Hao Wang 1, 2 , Xiaojie Deng 1, 2 , Jiahao Liu 1, 2 , Chen Liu 1, 2 , Yonghao Li 1, 2 , Weiyi Fang 1, 2
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-04-03 , DOI: 10.1038/s41419-019-1457-9 Zixi Liang 1, 2 , Zhen Liu 2, 3 , Chao Cheng 2, 4 , Hao Wang 1, 2 , Xiaojie Deng 1, 2 , Jiahao Liu 1, 2 , Chen Liu 1, 2 , Yonghao Li 1, 2 , Weiyi Fang 1, 2
Affiliation
|
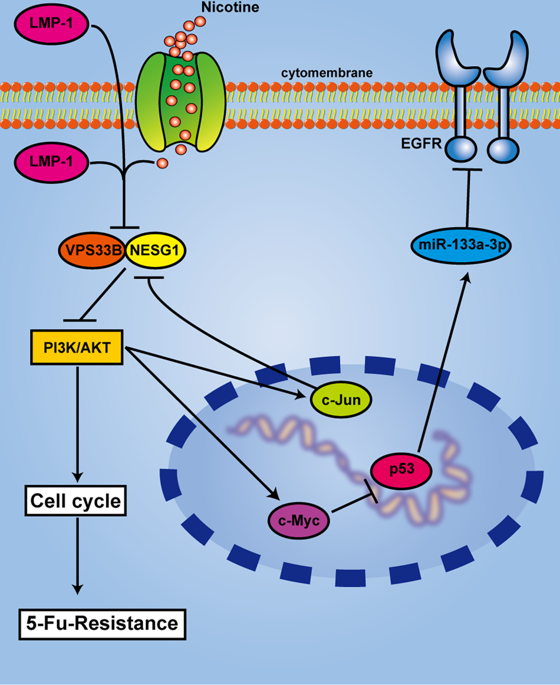
The vacuolar protein sorting 33B (VPS33B) was rarely reported in malignant tumors. In this research, we demonstrated that overexpression of VPS33B inhibited proliferation and chemoresistance to fluorouracil (5-FU) in nasopharyngeal carcinoma (NPC) in vivo and in vitro. Mechanistic analysis confirmed that overexpression of VPS33B modulated EGFR/PI3K/AKT/c-Myc/P53 signaling to arrest the cell cycle at G1/S phase. In addition, miR-133a-3p, a tumor-suppressive miRNA, was induced by P53 and directly targeted the EGFR/PI3K/AKT/c-Myc/P53 signaling and thus formed a negative feedback loop. Furthermore, another tumor suppressor, NESG1, interacted with VPS33B by colocalizing in the cytoplasm. The knockdown of NESG1 reversed the inhibitory effects of the overexpression of VPS33B in NPC cells by downregulating the PI3K/AKT/c-Jun-mediated transcription repression. Surprisingly, VPS33B was downregulated in the nicotine-treated and LMP-1-overexpressing NPC cells by targeting PI3K/AKT/c-Jun-mediated signaling. In addition, patients with higher VPS33B expression had a longer overall survival. Our study is the first to demonstrate that VPS33B is negatively regulated by LMP-1 and nicotine and thus suppresses the proliferation of NPC cells by interacting with NESG1 to regulate EGFR/PI3K/AKT/c-Myc/P53/miR-133a-3p signaling in NPC cells.
中文翻译:

VPS33B与NESG1相互作用以调节EGFR / PI3K / AKT / c-Myc / P53 / miR-133a-3p信号传导并诱导鼻咽癌中的5-氟尿嘧啶敏感性。
在恶性肿瘤中很少报道了液泡蛋白分选33B(VPS33B)。在这项研究中,我们证明了VPS33B的过表达在体内和体外抑制鼻咽癌(NPC)的增殖和对氟尿嘧啶(5-FU)的化学耐药性。机理分析证实,VPS33B的过表达调节了EGFR / PI3K / AKT / c-Myc / P53信号转导,使细胞周期停滞在G1 / S期。此外,抑癌的miRNA miR-133a-3p被P53诱导并直接靶向EGFR / PI3K / AKT / c-Myc / P53信号传导,从而形成了一个负反馈环。此外,另一种肿瘤抑制因子NESG1通过共定位在细胞质中与VPS33B相互作用。NESG1的敲低通过下调PI3K / AKT / c-Jun介导的转录抑制作用,逆转了NPC细胞中VPS33B过表达的抑制作用。出人意料的是,通过靶向PI3K / AKT / c-Jun介导的信号传导,VPS33B在尼古丁治疗和LMP-1过表达的NPC细胞中被下调。此外,VPS33B表达较高的患者的总生存期更长。我们的研究首次证明VPS33B受LMP-1和尼古丁负调节,从而通过与NESG1相互作用调节EGFR / PI3K / AKT / c-Myc / P53 / miR-133a-3p信号传导而抑制NPC细胞的增殖。在NPC细胞中 VPS33B表达较高的患者的总生存期更长。我们的研究首次证明VPS33B受LMP-1和尼古丁负调节,从而通过与NESG1相互作用调节EGFR / PI3K / AKT / c-Myc / P53 / miR-133a-3p信号传导而抑制NPC细胞的增殖。在NPC细胞中 VPS33B表达较高的患者的总生存期更长。我们的研究首次证明VPS33B受LMP-1和尼古丁负调节,从而通过与NESG1相互作用调节EGFR / PI3K / AKT / c-Myc / P53 / miR-133a-3p信号传导而抑制NPC细胞的增殖。在NPC细胞中
更新日期:2019-04-03
中文翻译:

VPS33B与NESG1相互作用以调节EGFR / PI3K / AKT / c-Myc / P53 / miR-133a-3p信号传导并诱导鼻咽癌中的5-氟尿嘧啶敏感性。
在恶性肿瘤中很少报道了液泡蛋白分选33B(VPS33B)。在这项研究中,我们证明了VPS33B的过表达在体内和体外抑制鼻咽癌(NPC)的增殖和对氟尿嘧啶(5-FU)的化学耐药性。机理分析证实,VPS33B的过表达调节了EGFR / PI3K / AKT / c-Myc / P53信号转导,使细胞周期停滞在G1 / S期。此外,抑癌的miRNA miR-133a-3p被P53诱导并直接靶向EGFR / PI3K / AKT / c-Myc / P53信号传导,从而形成了一个负反馈环。此外,另一种肿瘤抑制因子NESG1通过共定位在细胞质中与VPS33B相互作用。NESG1的敲低通过下调PI3K / AKT / c-Jun介导的转录抑制作用,逆转了NPC细胞中VPS33B过表达的抑制作用。出人意料的是,通过靶向PI3K / AKT / c-Jun介导的信号传导,VPS33B在尼古丁治疗和LMP-1过表达的NPC细胞中被下调。此外,VPS33B表达较高的患者的总生存期更长。我们的研究首次证明VPS33B受LMP-1和尼古丁负调节,从而通过与NESG1相互作用调节EGFR / PI3K / AKT / c-Myc / P53 / miR-133a-3p信号传导而抑制NPC细胞的增殖。在NPC细胞中 VPS33B表达较高的患者的总生存期更长。我们的研究首次证明VPS33B受LMP-1和尼古丁负调节,从而通过与NESG1相互作用调节EGFR / PI3K / AKT / c-Myc / P53 / miR-133a-3p信号传导而抑制NPC细胞的增殖。在NPC细胞中 VPS33B表达较高的患者的总生存期更长。我们的研究首次证明VPS33B受LMP-1和尼古丁负调节,从而通过与NESG1相互作用调节EGFR / PI3K / AKT / c-Myc / P53 / miR-133a-3p信号传导而抑制NPC细胞的增殖。在NPC细胞中


















































 京公网安备 11010802027423号
京公网安备 11010802027423号