Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Detection of Trinitrophenol by Amphiphilic Dimethylaminopyridine-Appended Zn(II)phthalocyanines at the Near-Infrared Region.
ACS Omega ( IF 3.7 ) Pub Date : 2019-04-03 , DOI: 10.1021/acsomega.8b02394 S Kasthuri 1 , Pratiksha Gawas 1 , Samarendra Maji 1 , N Veeraiah 2 , N Venkatramaiah 1
ACS Omega ( IF 3.7 ) Pub Date : 2019-04-03 , DOI: 10.1021/acsomega.8b02394 S Kasthuri 1 , Pratiksha Gawas 1 , Samarendra Maji 1 , N Veeraiah 2 , N Venkatramaiah 1
Affiliation
|
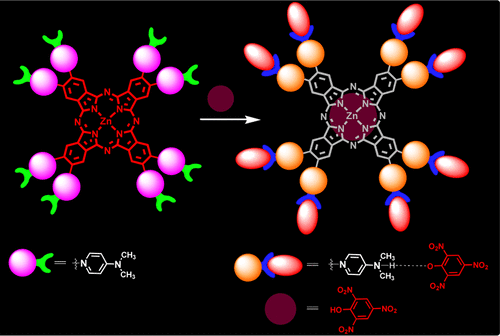
Novel amphiphilic Zn(II)phthalocyanines (ZnPcs) peripherally substituted with four and eight dimethylaminopyridinium units (ZnPc1 and ZnPc2) were synthesized by cyclotetramerization of the corresponding phthalonitriles. The effect of aggregation and photophysical (fluorescence quantum yields and lifetimes) and photochemical (singlet oxygen generation and photodegradation under light irradiation) properties was investigated. The chemosensing ability of ZnPcs toward explosive nitroaromatic compounds was explored in aqueous medium. This study demonstrates that ZnPc1 and ZnPc2 show fluorescence quenching behavior upon interaction with different nitro analytes and show unprecedented selectivity toward 2,4,6-trinitrophenol with a limit of detection (LOD) of 0.7-1.1 ppm with a high quenching rate constant (K sv) of 1.6-2.02 × 105. The near-infrared (NIR) fluorescence in thin films was quenched efficiently because of the photoinduced electron-transfer process through strong intermolecular π-π and electrostatic interactions. The sensing process is highly reversible and free from the interference of other commonly encountered nitro analytes. Further, experiments were performed to demonstrate the use of ZnPcs as efficient heterogeneous photocatalysts in the reduction of nitro explosives. The smart dual performance of multicharged ZnPcs in aqueous media quantifies them as attractive candidates in developing sensor materials at the NIR region and to possibly convert the toxic explosives into useful scaffolds. These results provide an interesting perspective toward elaboration of stable fluorescent systems for the selective sensing behavior of nitro explosives and their facile heterogeneous catalytic behavior in the reduction reactions.
中文翻译:

通过两亲性二甲基氨基吡啶附加的近红外区域的Zn(II)酞菁选择性检测三硝基苯酚。
通过将相应的邻苯二甲腈进行环四聚反应,合成了被四个和八个二甲基氨基吡啶鎓单元(ZnPc1和ZnPc2)外围取代的新型两亲性Zn(II)酞菁(ZnPcs)。研究了聚集和光物理性质(荧光量子产率和寿命)和光化学性质(光照射下单氧的产生和光降解)的影响。在水介质中探索了ZnPcs对爆炸性硝基芳族化合物的化学传感能力。这项研究表明ZnPc1和ZnPc2在与不同的硝基分析物相互作用时显示出荧光猝灭行为,并显示出对2,4,6-三硝基苯酚的前所未有的选择性,检测限(LOD)为0.7-1.1 ppm,且猝灭速率常数高(K sv)为1.6-2.02×105。薄膜中的近红外(NIR)荧光由于强分子间π-π和静电相互作用的光诱导电子转移过程而被有效淬灭。传感过程具有高度可逆性,并且不受其他常见的硝基分析物的干扰。此外,进行了实验以证明在还原硝基炸药中使用ZnPcs作为有效的多相光催化剂。水性介质中多电荷ZnPcs的智能双重性能将其量化为在NIR区域开发传感器材料中的引人注目的候选物,并有可能将有毒爆炸物转化为有用的支架。
更新日期:2019-04-03
中文翻译:

通过两亲性二甲基氨基吡啶附加的近红外区域的Zn(II)酞菁选择性检测三硝基苯酚。
通过将相应的邻苯二甲腈进行环四聚反应,合成了被四个和八个二甲基氨基吡啶鎓单元(ZnPc1和ZnPc2)外围取代的新型两亲性Zn(II)酞菁(ZnPcs)。研究了聚集和光物理性质(荧光量子产率和寿命)和光化学性质(光照射下单氧的产生和光降解)的影响。在水介质中探索了ZnPcs对爆炸性硝基芳族化合物的化学传感能力。这项研究表明ZnPc1和ZnPc2在与不同的硝基分析物相互作用时显示出荧光猝灭行为,并显示出对2,4,6-三硝基苯酚的前所未有的选择性,检测限(LOD)为0.7-1.1 ppm,且猝灭速率常数高(K sv)为1.6-2.02×105。薄膜中的近红外(NIR)荧光由于强分子间π-π和静电相互作用的光诱导电子转移过程而被有效淬灭。传感过程具有高度可逆性,并且不受其他常见的硝基分析物的干扰。此外,进行了实验以证明在还原硝基炸药中使用ZnPcs作为有效的多相光催化剂。水性介质中多电荷ZnPcs的智能双重性能将其量化为在NIR区域开发传感器材料中的引人注目的候选物,并有可能将有毒爆炸物转化为有用的支架。





























 京公网安备 11010802027423号
京公网安备 11010802027423号