Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytical Specificity, Reaction Mechanisms, and Conformational Changes during Catalysis of the Recombinant SUMO (+)-Zizaene Synthase from Chrysopogon zizanioides
ACS Omega ( IF 3.7 ) Pub Date : 2019-04-03 00:00:00 , DOI: 10.1021/acsomega.9b00242 Francisco Aguilar 1, 2 , Stephan Hartwig 1 , Thomas Scheper 1 , Sascha Beutel 1
ACS Omega ( IF 3.7 ) Pub Date : 2019-04-03 00:00:00 , DOI: 10.1021/acsomega.9b00242 Francisco Aguilar 1, 2 , Stephan Hartwig 1 , Thomas Scheper 1 , Sascha Beutel 1
Affiliation
|
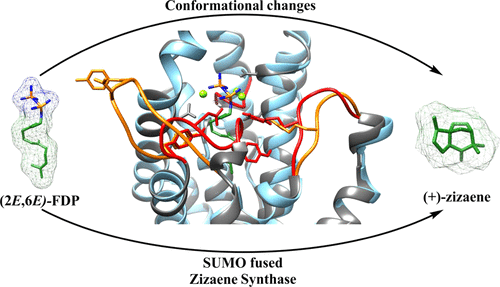
Zizaene synthase (ZS) from Chrysopogon zizanioides (Poaceae) is the critical enzyme in the biosynthesis of the fragrant sesquiterpene khusimol, a major component of the vetiver essential oil used widely by the cosmetic industry. As reported previously, we heterologously and successfully expressed the active ZS with a small ubiquitin-related modifier (SUMO) fusion domain. In this study, we report the optimization of reaction conditions and determination of enzyme kinetics of ZS. Moreover, we investigate the catalytic specificity and reaction mechanisms with the ubiquitous (2E,6E)-farnesyl diphosphate (FDP) and with C10 and C15 prenyl diphosphate isomers. Catalytic promiscuity occurs with monoterpene substrates generating eight products that comprise acyclic, cyclic, and hydroxylated monoterpenes. In contrast, ZS is a high-fidelity terpene cyclase when used with C15 isomer substrates, yielding as major products (Z)-β-farnesene (100%) for (2E,6Z)-FDP and (+)-zizaene (81.7%), β-acoradiene (12.8%), and (E)-β-farnesene (5.5%) for (2Z,6E)-FDP. Cyclization of the ubiquitous substrate (2E,6E)-FDP demonstrates a higher catalytic specificity, whereas the reaction proceeds via the acorenyl cation that generates (+)-zizaene (91.5%) and β-acoradiene (8.5%). Furthermore, catalytic specificity with (2E,6E)-FDP was stable in reactions tested at distinct pH and temperatures, suggesting a stable and efficient closed conformation of the active site during catalysis. To understand such stability, open and closed structural conformations of ZS were modeled in silico and revealed putative residues in the active site and in the A-C and J-K surrounding loops, which could explain the high fidelity of ZS.
中文翻译:

棉铃虫(Chrysopogon zizanioides)的重组SUMO(+)-Zizaene合酶催化过程中的催化特异性,反应机理和构象变化
香茅(Chrysopogon zizanioides)(禾本科)中的梓烯合酶(ZS)是香叶倍半萜香气酚的生物合成中的关键酶,香叶倍半萜香气酚是化妆品工业广泛使用的香根草精油的主要成分。如先前所报道,我们异源成功地用小泛素相关修饰子(SUMO)融合域表达了活性ZS。在这项研究中,我们报告了反应条件的优化和ZS酶动力学的确定。此外,我们研究了与普遍存在的(2 E,6 E)-法呢基二磷酸酯(FDP)以及C 10和C 15的催化特异性和反应机理异戊二烯基二磷酸异构体。单萜底物发生催化混杂,产生八种包含无环,环状和羟基化单萜的产物。相反,当与C 15异构体底物一起使用时,ZS是一种高保真萜烯环化酶,可作为(2 E,6 Z)-FDP和(+)-zizaene的主要产物(Z)-β-法呢烯(100%)(2 Z,6 E)-FDP的含量为(81.7%),β-二十碳烯(12.8%)和(E)-β-法呢烯(5.5%)。普遍存在的底物的环化(2 E,6 E)-FDP表现出更高的催化特异性,而反应则通过抗坏血酸阳离子进行,生成(+)-za氮烯(91.5%)和β-二十碳二烯(8.5%)。此外,在不同的pH和温度下测试的反应中,用(2 E,6 E)-FDP催化的特异性是稳定的,这表明在催化过程中活性位点具有稳定而有效的闭合构象。为了了解这种稳定性,在计算机上对ZS的开放和封闭结构构象进行了建模,并揭示了活性位点以及AC和JK周围环中的假定残基,这可以解释ZS的高保真度。
更新日期:2019-04-03
中文翻译:

棉铃虫(Chrysopogon zizanioides)的重组SUMO(+)-Zizaene合酶催化过程中的催化特异性,反应机理和构象变化
香茅(Chrysopogon zizanioides)(禾本科)中的梓烯合酶(ZS)是香叶倍半萜香气酚的生物合成中的关键酶,香叶倍半萜香气酚是化妆品工业广泛使用的香根草精油的主要成分。如先前所报道,我们异源成功地用小泛素相关修饰子(SUMO)融合域表达了活性ZS。在这项研究中,我们报告了反应条件的优化和ZS酶动力学的确定。此外,我们研究了与普遍存在的(2 E,6 E)-法呢基二磷酸酯(FDP)以及C 10和C 15的催化特异性和反应机理异戊二烯基二磷酸异构体。单萜底物发生催化混杂,产生八种包含无环,环状和羟基化单萜的产物。相反,当与C 15异构体底物一起使用时,ZS是一种高保真萜烯环化酶,可作为(2 E,6 Z)-FDP和(+)-zizaene的主要产物(Z)-β-法呢烯(100%)(2 Z,6 E)-FDP的含量为(81.7%),β-二十碳烯(12.8%)和(E)-β-法呢烯(5.5%)。普遍存在的底物的环化(2 E,6 E)-FDP表现出更高的催化特异性,而反应则通过抗坏血酸阳离子进行,生成(+)-za氮烯(91.5%)和β-二十碳二烯(8.5%)。此外,在不同的pH和温度下测试的反应中,用(2 E,6 E)-FDP催化的特异性是稳定的,这表明在催化过程中活性位点具有稳定而有效的闭合构象。为了了解这种稳定性,在计算机上对ZS的开放和封闭结构构象进行了建模,并揭示了活性位点以及AC和JK周围环中的假定残基,这可以解释ZS的高保真度。































 京公网安备 11010802027423号
京公网安备 11010802027423号