Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
抑郁症中连通性的改变:GABA 和谷氨酸神经递质的缺乏和新疗法的逆转
Neuron ( IF 14.7 ) Pub Date : 2019-04-03 , DOI: 10.1016/j.neuron.2019.03.013
Ronald S Duman 1 , Gerard Sanacora 1 , John H Krystal 1
Affiliation
|
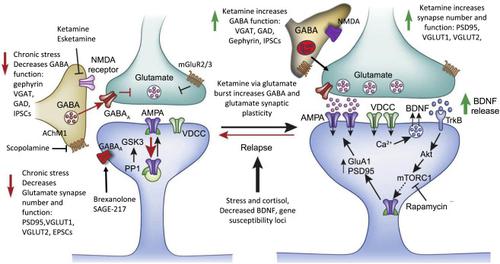
抑郁症和压力相关疾病的病理生理学和治疗机制尚不清楚,但对抑郁症患者和啮齿动物模型的研究已开始产生有希望的见解。这些研究表明,抑郁症和慢性压力暴露会导致与抑郁症有关的皮质和边缘脑区域的神经元萎缩,脑成像研究表明抑郁症患者大脑的连接性和网络功能发生了改变。对这些改变的神经生物学基础的研究集中在主要的兴奋性谷氨酸神经元和抑制性 GABA 中间神经元上。他们证明了两种主要神经元类型的结构、功能和神经化学缺陷,可能导致皮质和海马区域信号完整性退化。这些变化背后的分子机制尚未确定,但被认为与应激诱导的兴奋性毒性作用以及肾上腺糖皮质激素和炎症细胞因子以及其他环境因素升高有关。转录组学研究开始证明重要的性别差异,并与基因组研究一起开始揭示精神分裂症和双相情感障碍风险和病理生理机制的重叠和独特性机制领域。这些研究还涉及 GABA 和谷氨酸功能障碍以及免疫机制。虽然目前的抗抑郁药存在显着的时滞和疗效限制,但针对谷氨酸和 GABA 系统的新型速效药物可以解决这些问题,并为这种广泛存在的衰弱性疾病提供卓越的治疗干预措施。

"点击查看英文标题和摘要"

































 京公网安备 11010802027423号
京公网安备 11010802027423号