当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Noncoded Amino Acids in de Novo Metalloprotein Design: Controlling Coordination Number and Catalysis.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-04-01 00:00:00 , DOI: 10.1021/acs.accounts.9b00032 Karl J Koebke 1 , Vincent L Pecoraro 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-04-01 00:00:00 , DOI: 10.1021/acs.accounts.9b00032 Karl J Koebke 1 , Vincent L Pecoraro 1
Affiliation
|
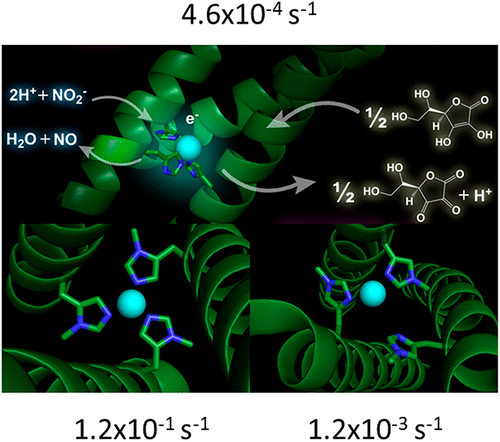
The relationship between structure and function has long been one of the major points of investigation in Biophysics. Understanding how much, or how little, of a protein’s often complicated structure is necessary for its function can lead to directed therapeutic strategies and would allow one to design proteins for specific desired functions. Studying protein function by de novo design builds the functionality from the ground up in a completely unrelated and noncoded protein scaffold. Our lab has used this strategy to study heavy and transition metal binding within the TRI family of three stranded coiled coil (3SCC) constructs to understand coordination geometry and metalloenzyme catalytic control within a protein environment. These peptides contain hydrophobic layers within the interior of the 3SCC, which one can mutate to metal binding residues to create a minimal metal binding site, while solid phase synthesis allows our lab to easily incorporate a number of noncoded amino acids including d enantiomers of binding or secondary coordination sphere amino acids, penicillamine, or methylated versions of histidine.
中文翻译:

新生金属蛋白设计中的非编码氨基酸:控制配位数和催化。
长期以来,结构与功能之间的关系一直是生物物理学研究的重点之一。了解蛋白质通常需要多少结构才能正常发挥其功能,这可能会导致针对性的治疗策略,并使人们可以设计出具有特定所需功能的蛋白质。从头研究蛋白质功能设计在完全不相关且未编码的蛋白质支架中从头开始构建功能。我们的实验室已使用该策略研究了三链卷曲螺旋(3SCC)构造的TRI系列中的重金属和过渡金属结合,以了解蛋白质环境中的配位几何和金属酶催化控制。这些肽在3SCC内部包含疏水层,该层可以突变为金属结合残基以创建最小的金属结合位点,而固相合成使我们的实验室可以轻松地掺入许多非编码氨基酸,包括结合或结合的d个对映体。次要配位域氨基酸,青霉胺或组氨酸的甲基化形式。
更新日期:2019-04-01
中文翻译:

新生金属蛋白设计中的非编码氨基酸:控制配位数和催化。
长期以来,结构与功能之间的关系一直是生物物理学研究的重点之一。了解蛋白质通常需要多少结构才能正常发挥其功能,这可能会导致针对性的治疗策略,并使人们可以设计出具有特定所需功能的蛋白质。从头研究蛋白质功能设计在完全不相关且未编码的蛋白质支架中从头开始构建功能。我们的实验室已使用该策略研究了三链卷曲螺旋(3SCC)构造的TRI系列中的重金属和过渡金属结合,以了解蛋白质环境中的配位几何和金属酶催化控制。这些肽在3SCC内部包含疏水层,该层可以突变为金属结合残基以创建最小的金属结合位点,而固相合成使我们的实验室可以轻松地掺入许多非编码氨基酸,包括结合或结合的d个对映体。次要配位域氨基酸,青霉胺或组氨酸的甲基化形式。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号