当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Situ X-ray Absorption Spectroscopic Investigation of the Capacity Degradation Mechanism in Mg/S Batteries
Nano Letters ( IF 9.6 ) Pub Date : 2019-04-01 00:00:00 , DOI: 10.1021/acs.nanolett.8b05208 Yan Xu 1, 2 , Yifan Ye 3 , Shuyang Zhao 4 , Jun Feng 3 , Jia Li 4 , Hao Chen 5 , Ankun Yang 5 , Feifei Shi 5 , Lujie Jia 6 , Yang Wu 6 , Xiaoyun Yu 5 , Per-Anders Glans-Suzuki 3 , Yi Cui 5 , Jinghua Guo 3 , Yuegang Zhang 1, 6
Nano Letters ( IF 9.6 ) Pub Date : 2019-04-01 00:00:00 , DOI: 10.1021/acs.nanolett.8b05208 Yan Xu 1, 2 , Yifan Ye 3 , Shuyang Zhao 4 , Jun Feng 3 , Jia Li 4 , Hao Chen 5 , Ankun Yang 5 , Feifei Shi 5 , Lujie Jia 6 , Yang Wu 6 , Xiaoyun Yu 5 , Per-Anders Glans-Suzuki 3 , Yi Cui 5 , Jinghua Guo 3 , Yuegang Zhang 1, 6
Affiliation
|
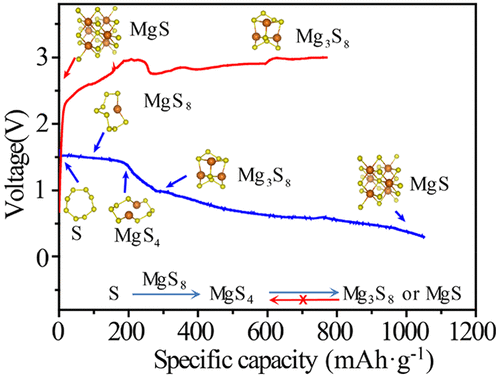
The Mg/S battery is attractive because of its high theoretical energy density and the abundance of Mg and S on the earth. However, its development is hindered by the lack of understanding to the underlying electrochemical reaction mechanism of its charge–discharge processes. Here, using a unique in situ X-ray absorption spectroscopic tool, we systematically study the reaction pathways of the Mg/S cells in Mg(HMDS)2–AlCl3 electrolyte. We find that the capacity degradation is mainly due to the formation of irreversible discharge products, such as MgS and Mg3S8, through a direct electrochemical deposition or a chemical disproportionation of intermediate polysulfide. In light of the fundamental understanding, we propose to use TiS2 as a catalyst to activate the irreversible reaction of low-order MgSx and MgS, which results in an increased discharging capacity up to 900 mAh·g–1 and a longer cycling life.
中文翻译:

Mg / S电池容量衰减机理的原位X射线吸收光谱研究
Mg / S电池之所以具有吸引力,是因为其理论能量密度高,并且地球上的Mg和S含量很高。但是,由于对其充放电过程的基本电化学反应机理缺乏了解,阻碍了它的发展。在这里,我们使用独特的原位X射线吸收光谱仪,系统地研究了Mg(HMDS)2 -AlCl 3电解质中Mg / S细胞的反应途径。我们发现容量下降主要是由于不可逆放电产物的形成,例如MgS和Mg 3 S 8通过直接电化学沉积或中间多硫化物的化学歧化来实现。根据基本理解,我们建议使用TiS 2作为催化剂来激活低级MgS x和MgS的不可逆反应,从而使放电容量增加至900 mAh·g –1,并延长循环寿命。
更新日期:2019-04-01
中文翻译:

Mg / S电池容量衰减机理的原位X射线吸收光谱研究
Mg / S电池之所以具有吸引力,是因为其理论能量密度高,并且地球上的Mg和S含量很高。但是,由于对其充放电过程的基本电化学反应机理缺乏了解,阻碍了它的发展。在这里,我们使用独特的原位X射线吸收光谱仪,系统地研究了Mg(HMDS)2 -AlCl 3电解质中Mg / S细胞的反应途径。我们发现容量下降主要是由于不可逆放电产物的形成,例如MgS和Mg 3 S 8通过直接电化学沉积或中间多硫化物的化学歧化来实现。根据基本理解,我们建议使用TiS 2作为催化剂来激活低级MgS x和MgS的不可逆反应,从而使放电容量增加至900 mAh·g –1,并延长循环寿命。































 京公网安备 11010802027423号
京公网安备 11010802027423号