当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
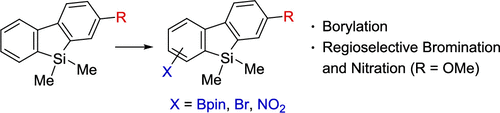
Regioselective Functionalization of 9,9-Dimethyl-9-silafluorenes by Borylation, Bromination, and Nitration
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-04-02 00:00:00 , DOI: 10.1021/acs.joc.9b00598 Masahito Murai 1 , Naoki Nishinaka 1 , Mizuki Kimura 1 , Kazuhiko Takai 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-04-02 00:00:00 , DOI: 10.1021/acs.joc.9b00598 Masahito Murai 1 , Naoki Nishinaka 1 , Mizuki Kimura 1 , Kazuhiko Takai 1
Affiliation
|
Despite the utility of 9-silafluorenes as functional materials and as building blocks, methods for efficient functionalization of their backbone are rare, probably because of the presence of easily cleavable C–Si bonds. Although controlling the regioselectivity of iridium-catalyzed direct borylation of C–H bonds is difficult, we found that bromination and nitration of 2-methoxy-9-silafluorene under mild conditions occurred predominantly at the electron-rich position. The resulting product having methoxy and bromo groups can be utilized as a building block for the synthesis of unsymmetrically substituted 9-silafluorene-containing π-conjugated molecules.
中文翻译:

通过硼化,溴化和硝化作用对9,9-二甲基-9-硅芴进行区域选择性官能化
尽管使用9硅芴作为功能材料和结构单元,但对其骨架进行有效功能化的方法却很少,这可能是由于存在易于裂解的C-Si键所致。尽管很难控制铱催化的C–H键直接硼化的区域选择性,但我们发现2-甲氧基-9-硅芴在温和条件下的溴化和硝化主要发生在富电子位置。具有甲氧基和溴基的所得产物可用作合成不对称取代的含9-硅芴的π-共轭分子的结构单元。
更新日期:2019-04-02
中文翻译:

通过硼化,溴化和硝化作用对9,9-二甲基-9-硅芴进行区域选择性官能化
尽管使用9硅芴作为功能材料和结构单元,但对其骨架进行有效功能化的方法却很少,这可能是由于存在易于裂解的C-Si键所致。尽管很难控制铱催化的C–H键直接硼化的区域选择性,但我们发现2-甲氧基-9-硅芴在温和条件下的溴化和硝化主要发生在富电子位置。具有甲氧基和溴基的所得产物可用作合成不对称取代的含9-硅芴的π-共轭分子的结构单元。








































 京公网安备 11010802027423号
京公网安备 11010802027423号