当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complexation of Ammonia Boranes with Al3+
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-04-02 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02630 Iurii Dovgaliuk 1, 2 , Kasper T. Møller 1, 3 , Koen Robeyns 1 , Véronique Louppe 1 , Torben R. Jensen 3 , Yaroslav Filinchuk 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-04-02 00:00:00 , DOI: 10.1021/acs.inorgchem.8b02630 Iurii Dovgaliuk 1, 2 , Kasper T. Møller 1, 3 , Koen Robeyns 1 , Véronique Louppe 1 , Torben R. Jensen 3 , Yaroslav Filinchuk 1
Affiliation
|
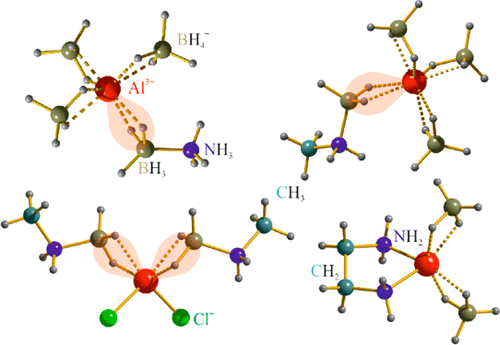
Ammonia borane, NH3BH3 (AB), is very attractive for hydrogen storage; however, it dehydrogenates exothermally, producing a mixture of polymeric products with limited potential for direct rehydrogenation. Recently, it was shown that AB complexed with Al3+ in Al(BH4)3·AB endothermically dehydrogenates to a single product identified as Al(BH4)3·NHBH, with the potential for direct rehydrogenation of AB. Here we explore the reactivity of AB-derived RNH2BH3 (R = −CH3, −CH2−) with AlX3 salts (X = BH4–, Cl–), aiming to extend the series to different anions and to enlarge the stability window for Al(BH4)3·NRBH. Three novel complexes were identified: Al(BH4)3·CH3NH2BH3 having a molecular structure similar to that of Al(BH4)3·AB but different dehydrogenation properties, as well as [Al(CH3NH2BH3)2Cl2][AlCl4] and [Al(NH2CH2CH2NH2)(BH4)2][Al(BH4)4], rare examples of Al3+ making part of the cations and anions simultaneously. The latter compounds are of interest in the design of novel electrolytes for Al-based batteries. The coordination of two ABs to a single Al atom opens a route to materials with higher hydrogen content.
中文翻译:

氨硼烷与Al 3+的络合
氨硼烷NH 3 BH 3(AB)对于储氢非常有吸引力。然而,它放热地脱氢,产生了直接再氢化潜力有限的聚合物产物的混合物。最近,显示出与Al (BH 4)3 ·AB中的Al 3+络合的AB吸热脱氢为单一产物,鉴定为Al(BH 4)3 ·NHBH,具有直接AB再氢化的潜力。在这里,我们探讨了AB衍生的RNH 2 BH 3(R = -CH 3,-CH 2-)与AlX 3盐(X = BH 4 –,Cl –),旨在将系列扩展至不同的阴离子,并扩大Al(BH 4)3 ·NRBH的稳定性窗口。鉴定出三种新颖的配合物:具有与Al(BH 4)3 ·AB相似的分子结构但具有不同的脱氢性能的Al(BH 4)3 ·CH 3 NH 2 BH 3以及[Al(CH 3 NH 2) BH 3)2 Cl 2 ] [AlCl 4 ]和[Al(NH 2 CH 2 CH 2 NH 2)(BH 4)2 ] [Al(BH 4)4 ],同时成为阳离子和阴离子一部分的Al 3+的稀有例子。后一种化合物在用于铝基电池的新型电解质的设计中是令人感兴趣的。两个AB与单个Al原子的配位为氢含量更高的材料开辟了一条途径。
更新日期:2019-04-02
中文翻译:

氨硼烷与Al 3+的络合
氨硼烷NH 3 BH 3(AB)对于储氢非常有吸引力。然而,它放热地脱氢,产生了直接再氢化潜力有限的聚合物产物的混合物。最近,显示出与Al (BH 4)3 ·AB中的Al 3+络合的AB吸热脱氢为单一产物,鉴定为Al(BH 4)3 ·NHBH,具有直接AB再氢化的潜力。在这里,我们探讨了AB衍生的RNH 2 BH 3(R = -CH 3,-CH 2-)与AlX 3盐(X = BH 4 –,Cl –),旨在将系列扩展至不同的阴离子,并扩大Al(BH 4)3 ·NRBH的稳定性窗口。鉴定出三种新颖的配合物:具有与Al(BH 4)3 ·AB相似的分子结构但具有不同的脱氢性能的Al(BH 4)3 ·CH 3 NH 2 BH 3以及[Al(CH 3 NH 2) BH 3)2 Cl 2 ] [AlCl 4 ]和[Al(NH 2 CH 2 CH 2 NH 2)(BH 4)2 ] [Al(BH 4)4 ],同时成为阳离子和阴离子一部分的Al 3+的稀有例子。后一种化合物在用于铝基电池的新型电解质的设计中是令人感兴趣的。两个AB与单个Al原子的配位为氢含量更高的材料开辟了一条途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号