当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deconvoluting the Molecular Control of Binding and Signaling at the Amylin 3 Receptor: RAMP3 Alters Signal Propagation through Extracellular Loops of the Calcitonin Receptor
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-03-25 , DOI: 10.1021/acsptsci.9b00010 Vi Pham 1 , Yue Zhu 2, 3 , Emma Dal Maso 1 , Christopher A Reynolds 4 , Giuseppe Deganutti 4 , Silvia Atanasio 4 , Caroline A Hick 1 , Dehua Yang 2 , Arthur Christopoulos 1 , Debbie L Hay 5 , Sebastian G B Furness 1 , Ming-Wei Wang 2, 3 , Denise Wootten 1 , Patrick M Sexton 1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2019-03-25 , DOI: 10.1021/acsptsci.9b00010 Vi Pham 1 , Yue Zhu 2, 3 , Emma Dal Maso 1 , Christopher A Reynolds 4 , Giuseppe Deganutti 4 , Silvia Atanasio 4 , Caroline A Hick 1 , Dehua Yang 2 , Arthur Christopoulos 1 , Debbie L Hay 5 , Sebastian G B Furness 1 , Ming-Wei Wang 2, 3 , Denise Wootten 1 , Patrick M Sexton 1
Affiliation
|
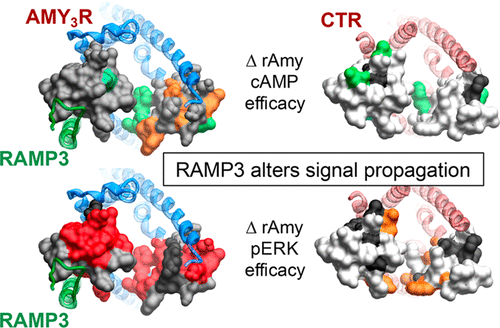
Amylin is coexpressed with insulin in pancreatic islet β-cells and has potent effects on gastric emptying and food intake. The effect of amylin on satiation has been postulated to involve AMY3 receptors (AMY3R) that are heteromers of the calcitonin receptor (CTR) and receptor activity-modifying protein 3 (RAMP3). Understanding the molecular control of signaling through the AMY3R is thus important for peptide drug targeting of this receptor. We have previously used alanine scanning mutagenesis to study the contribution of the extracellular surface of the CTR to binding and signaling initiated by calcitonin (CT) and related peptides (Dal Maso, E., et al. (2019) The molecular control of calcitonin receptor signaling. ACS Pharmacol. Transl. Sci.2, 31–51). That work revealed ligand- and pathway-specific effects of mutation, with extracellular loops (ECLs) 2 and 3 particularly important in the distinct propagation of signaling mediated by individual peptides. In the current study, we have used equivalent alanine scanning of ECL2 and ECL3 of the CTR in the context of coexpression with RAMP3 to form AMY3Rs, to examine functional affinity and efficacy of peptides in cAMP accumulation and extracellular signal-regulated kinase (ERK) phosphorylation (pERK). The effect of mutation was determined on representatives of the three major distinct classes of CT peptide, salmon CT (sCT), human CT (hCT), and porcine CT (pCT), as well as rat amylin (rAmy) or human α-CGRP (calcitonin gene-related peptide, hCGRP) whose potency is enhanced by RAMP interaction. We demonstrate that the dynamic nature of CTR ECL2 and ECL3 in propagation of signaling is fundamentally altered when complexed with RAMP3 to form the AMY3R, despite only having predicted direct interactions with ECL2. Moreover, the work shows that the role of these loops in receptor signaling is highly peptide dependent, illustrating that even subtle changes to peptide sequence may change signaling output downstream of the receptor.
中文翻译:

解卷积胰淀素 3 受体结合和信号传导的分子控制:RAMP3 通过降钙素受体的细胞外环改变信号传播
胰淀素与胰岛 β 细胞中的胰岛素共表达,对胃排空和食物摄入具有有效影响。据推测,胰淀素对饱腹感的影响涉及 AMY 3受体 (AMY 3 R),它们是降钙素受体 (CTR) 和受体活性修饰蛋白 3 (RAMP3) 的异聚体。因此,了解 AMY 3 R 信号传导的分子控制对于靶向该受体的肽药物非常重要。我们之前使用丙氨酸扫描诱变来研究 CTR 细胞外表面对降钙素 (CT) 和相关肽引发的结合和信号传导的贡献(Dal Maso, E., et al . (2019) 降钙素受体的分子控制ACS 药理学翻译。2,31-51 ) 。这项工作揭示了突变的配体和通路特异性效应,其中细胞外环 (ECL) 2 和 3 在单个肽介导的信号传导的独特传播中尤其重要。在当前的研究中,我们在与 RAMP3 共表达形成 AMY 3 Rs 的背景下,对 CTR 的 ECL2 和 ECL3 使用等效丙氨酸扫描,以检查肽在 cAMP 积累和细胞外信号调节激酶 (ERK) 中的功能亲和力和功效。 ) 磷酸化 (pERK)。突变的影响是通过三种主要不同类别的 CT 肽代表来确定的:鲑鱼 CT (sCT)、人 CT (hCT) 和猪 CT (pCT),以及大鼠胰岛淀粉样多肽 (rAmy) 或人 α-CGRP (降钙素基因相关肽,hCGRP)其效力通过 RAMP 相互作用而增强。 我们证明,尽管仅预测了与 ECL2 的直接相互作用,但当与 RAMP3 复合形成 AMY 3 R 时,CTR ECL2 和 ECL3 在信号传播中的动态性质发生了根本改变。此外,该工作表明这些环在受体信号传导中的作用是高度肽依赖性的,这表明即使肽序列的细微变化也可能改变受体下游的信号传导输出。
更新日期:2019-05-23
中文翻译:

解卷积胰淀素 3 受体结合和信号传导的分子控制:RAMP3 通过降钙素受体的细胞外环改变信号传播
胰淀素与胰岛 β 细胞中的胰岛素共表达,对胃排空和食物摄入具有有效影响。据推测,胰淀素对饱腹感的影响涉及 AMY 3受体 (AMY 3 R),它们是降钙素受体 (CTR) 和受体活性修饰蛋白 3 (RAMP3) 的异聚体。因此,了解 AMY 3 R 信号传导的分子控制对于靶向该受体的肽药物非常重要。我们之前使用丙氨酸扫描诱变来研究 CTR 细胞外表面对降钙素 (CT) 和相关肽引发的结合和信号传导的贡献(Dal Maso, E., et al . (2019) 降钙素受体的分子控制ACS 药理学翻译。2,31-51 ) 。这项工作揭示了突变的配体和通路特异性效应,其中细胞外环 (ECL) 2 和 3 在单个肽介导的信号传导的独特传播中尤其重要。在当前的研究中,我们在与 RAMP3 共表达形成 AMY 3 Rs 的背景下,对 CTR 的 ECL2 和 ECL3 使用等效丙氨酸扫描,以检查肽在 cAMP 积累和细胞外信号调节激酶 (ERK) 中的功能亲和力和功效。 ) 磷酸化 (pERK)。突变的影响是通过三种主要不同类别的 CT 肽代表来确定的:鲑鱼 CT (sCT)、人 CT (hCT) 和猪 CT (pCT),以及大鼠胰岛淀粉样多肽 (rAmy) 或人 α-CGRP (降钙素基因相关肽,hCGRP)其效力通过 RAMP 相互作用而增强。 我们证明,尽管仅预测了与 ECL2 的直接相互作用,但当与 RAMP3 复合形成 AMY 3 R 时,CTR ECL2 和 ECL3 在信号传播中的动态性质发生了根本改变。此外,该工作表明这些环在受体信号传导中的作用是高度肽依赖性的,这表明即使肽序列的细微变化也可能改变受体下游的信号传导输出。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号