当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulating Reactivity and Selectivity of 2-Pyrone-Derived Bicyclic Lactones through Choice of Catalyst and Solvent
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-02-16 00:00:00 , DOI: 10.1021/acscatal.7b04311 Toni Pfennig 1 , Ashwin Chemburkar 1, 2 , Robert L. Johnson 1 , Matthew J. Ryan , Aaron J. Rossini , Matthew Neurock 1, 2 , Brent H. Shanks 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-02-16 00:00:00 , DOI: 10.1021/acscatal.7b04311 Toni Pfennig 1 , Ashwin Chemburkar 1, 2 , Robert L. Johnson 1 , Matthew J. Ryan , Aaron J. Rossini , Matthew Neurock 1, 2 , Brent H. Shanks 1
Affiliation
|
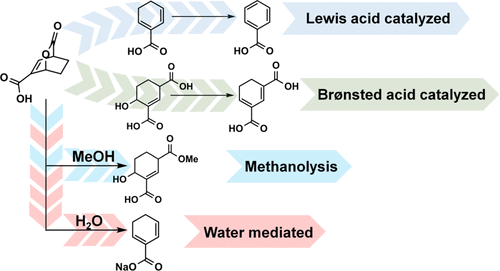
2-Pyrones, such as coumalic acid, are promising biobased molecules that through Diels–Alder reactions can provide access to a wide range of biobased chemicals, including molecules with functionality that are not easily accessible via conventional petrochemical routes. A complete reaction network and kinetic parameters for three individual diversification routes that start from a single bicyclic lactone produced via the Diels–Alder cycloaddition of coumalic acid and ethylene were examined experimentally and probed through complementary first-principle density functional theory (DFT) calculations, in situ nuclear magnetic resonance (NMR) spectroscopy, and thin film solid-state NMR spectroscopy. These experiments provide insights into the routes for several molecular structures from bicyclic lactones by leveraging Lewis or Brønsted acid catalysts to selectively alter the reaction pathway. The bicyclic lactone bridge can be decarboxylated to access dihydrobenzenes at a substantially reduced activation barrier using γ-Al2O3 as the catalyst or selectively ring-opened via Brønsted acids to yield 1,3-diacid six membered rings. DFT computations and microkinetic modeling in combination with experimental results provide molecular insights into the catalytically active sites on γ-Al2O3 and provide a general mechanism for the catalyzed bicyclic lactone decarboxylation in polar aprotic solvents, which involves CO2 extrusion as the kinetically relevant step. Solid-state NMR spectroscopy provides direct evidence of strong binding of the bicyclic lactone to the γ-Al2O3 surface, fully consistent with DFT simulation results and experimental reaction kinetics. In addition, the role of the solvent was examined and found to be an additional means to improve reaction rates and selectively produce alternative structures from the bicyclic intermediate. The rate of the decarboxylation reaction was increased dramatically by using water as the solvent whereas methanol acted as a nucleophile and selectively induced ring-opening, showing that both pathways are operative in the absence of catalyst. Taken together, the results demonstrate an approach for selective diversification of the coumalate platform to a range of molecules.
中文翻译:

通过选择催化剂和溶剂来调节2-吡喃酮衍生的双环内酯的反应性和选择性
2-吡喃酮,例如香豆酸,是有前途的生物基分子,通过Diels-Alder反应可以提供广泛的生物基化学物质的访问权,包括功能不易通过常规石化途径获得的分子。实验研究了三种单独的多样化路线的完整反应网络和动力学参数,这些路线是通过对苯二酸和乙烯的Diels-Alder环加成产生的单个双环内酯开始的,并通过互补的第一原理密度泛函理论(DFT)计算进行了探索。原位核磁共振(NMR)光谱和薄膜固态NMR光谱。这些实验通过利用路易斯或布朗斯台德酸催化剂来选择性地改变反应路径,从而为双环内酯的几种分子结构的路线提供了见识。双环内酯桥可使用γ-Al进行脱羧反应,从而在实质上降低的活化势垒下获得二氢苯2 O 3作为催化剂或通过布朗斯台德酸选择性地开环生成1,3-二酸六元环。DFT计算和与实验结果组合microkinetic建模提供分子见解上的催化活性位点的γ-Al 2 ö 3和提供一种用于在极性非质子溶剂中的催化的双环内酯脱羧,它涉及到CO的通用机制2挤出作为动力学相关步。固态NMR光谱法提供了强的双环内酯而形成的结合的直接证据的γ-Al 2 ö 3表面,与DFT模拟结果和实验反应动力学完全一致。另外,检查了溶剂的作用,发现该溶剂是提高反应速率并选择性地从双环中间体产生替代结构的另一种方法。通过使用水作为溶剂,脱羧反应的速率显着提高,而甲醇充当亲核试剂并选择性地引起开环,表明这两种途径在没有催化剂的情况下都是有效的。综上所述,结果证明了将香豆酸酯平台选择性多样化发展为一系列分子的方法。
更新日期:2018-02-16
中文翻译:

通过选择催化剂和溶剂来调节2-吡喃酮衍生的双环内酯的反应性和选择性
2-吡喃酮,例如香豆酸,是有前途的生物基分子,通过Diels-Alder反应可以提供广泛的生物基化学物质的访问权,包括功能不易通过常规石化途径获得的分子。实验研究了三种单独的多样化路线的完整反应网络和动力学参数,这些路线是通过对苯二酸和乙烯的Diels-Alder环加成产生的单个双环内酯开始的,并通过互补的第一原理密度泛函理论(DFT)计算进行了探索。原位核磁共振(NMR)光谱和薄膜固态NMR光谱。这些实验通过利用路易斯或布朗斯台德酸催化剂来选择性地改变反应路径,从而为双环内酯的几种分子结构的路线提供了见识。双环内酯桥可使用γ-Al进行脱羧反应,从而在实质上降低的活化势垒下获得二氢苯2 O 3作为催化剂或通过布朗斯台德酸选择性地开环生成1,3-二酸六元环。DFT计算和与实验结果组合microkinetic建模提供分子见解上的催化活性位点的γ-Al 2 ö 3和提供一种用于在极性非质子溶剂中的催化的双环内酯脱羧,它涉及到CO的通用机制2挤出作为动力学相关步。固态NMR光谱法提供了强的双环内酯而形成的结合的直接证据的γ-Al 2 ö 3表面,与DFT模拟结果和实验反应动力学完全一致。另外,检查了溶剂的作用,发现该溶剂是提高反应速率并选择性地从双环中间体产生替代结构的另一种方法。通过使用水作为溶剂,脱羧反应的速率显着提高,而甲醇充当亲核试剂并选择性地引起开环,表明这两种途径在没有催化剂的情况下都是有效的。综上所述,结果证明了将香豆酸酯平台选择性多样化发展为一系列分子的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号