当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Construction of 4H-Pyrano[3,2-b]indoles via Cinchonine-Catalyzed 1,4-Addition of 2-Ylideneoxindole with Malononitrile
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-03-28 00:00:00 , DOI: 10.1021/acs.joc.9b00430 Jin Zhou 1 , Biao Wang 1 , Xiang-Hong He 1 , Li Liu 1 , Jun Wu 1 , Jing Lu 1 , Cheng Peng 1 , Chao-Long Rao 1 , Bo Han 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-03-28 00:00:00 , DOI: 10.1021/acs.joc.9b00430 Jin Zhou 1 , Biao Wang 1 , Xiang-Hong He 1 , Li Liu 1 , Jun Wu 1 , Jing Lu 1 , Cheng Peng 1 , Chao-Long Rao 1 , Bo Han 1
Affiliation
|
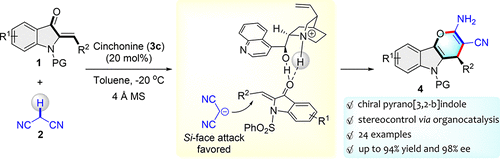
A highly enantioselective [4 + 2] annulation of 2-ylideneoxindole with malononitrile has been accomplished by cinchonine catalysis under mild conditions. The corresponding enantiomerically enriched 4H-pyrano[3,2-b]indoles were generated in moderate to high yields (up to 94%) with excellent enantioselectivities (up to 98% ee). To explain the stereoselectivity of the organocatalytic Michael-ammonization cascade, we also carried out the control experiments and proposed plausible transition-state models for the catalytic cycle based on the observed stereochemistry of the products. In addition, some of the products showed moderate antibacterial activity against S. aureus and S. epidermidis in vitro, which might be considered as a potential clue for the discovery of new antimicrobial agents.
中文翻译:

辛可宁催化2-亚硝基吲哚与丙二腈的1,4-加成反应不对称构建4 H-吡喃并[3,2- b ]吲哚
通过在温和条件下用辛可宁催化,可以实现2-亚硝基新吲哚与丙二腈的高度对映选择性[4 + 2]环化反应。相应的对映体富集的4 H-吡喃并[3,2- b ]吲哚以中等至高收率(高达94%)和优异的对映选择性(高达98%ee)生成。为了解释有机催化迈克尔-氨化级联反应的立体选择性,我们还进行了控制实验,并基于观察到的产物立体化学,提出了催化循环的合理过渡态模型。此外,某些产品在体外对金黄色葡萄球菌和表皮葡萄球菌具有中等程度的抗菌活性。,这可能被视为发现新的抗菌剂的潜在线索。
更新日期:2019-03-28
中文翻译:

辛可宁催化2-亚硝基吲哚与丙二腈的1,4-加成反应不对称构建4 H-吡喃并[3,2- b ]吲哚
通过在温和条件下用辛可宁催化,可以实现2-亚硝基新吲哚与丙二腈的高度对映选择性[4 + 2]环化反应。相应的对映体富集的4 H-吡喃并[3,2- b ]吲哚以中等至高收率(高达94%)和优异的对映选择性(高达98%ee)生成。为了解释有机催化迈克尔-氨化级联反应的立体选择性,我们还进行了控制实验,并基于观察到的产物立体化学,提出了催化循环的合理过渡态模型。此外,某些产品在体外对金黄色葡萄球菌和表皮葡萄球菌具有中等程度的抗菌活性。,这可能被视为发现新的抗菌剂的潜在线索。







































 京公网安备 11010802027423号
京公网安备 11010802027423号