Water Research ( IF 11.4 ) Pub Date : 2019-03-29 , DOI: 10.1016/j.watres.2019.03.078 Fubing Yao , Qi Yang , Yu Zhong , Xiaoyu Shu , Fei Chen , Jian Sun , Yinghao Ma , Zhiyan Fu , Dongbo Wang , Xiaoming Li
|
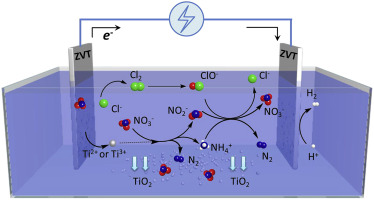
In this study, indirect electrochemical reduction with zero-valent titanium (ZVT) as anode successfully achieved the selective nitrate removal from simulated groundwater. The maximum nitrate removal efficiency and N2 selectivity reached to 83.4% and 78.5% after 12 h, respectively. Experimental results demonstrated that the gaseous by-products (NO and N2O) were negligible and the nitrate reduction process could be well depicted by pseudo-first-order kinetic model. Decreasing the pH value of electrolyte was favorable to electrical energy utilization efficiency and nitrate removal. The chloride ultimately showed inhibitory effects on electrochemical reduction of nitrate. During the electrochemical reaction, the ZVT lost electrons to generate the reducing agents (Ti3+ and Ti2+), which could afford electrons for nitrate reduction and form the solid by-products TiO2.4Cl0.2N0.1. A 2-stage strategy, indirect electrochemical reduction + hypochlorite treatment (pre-reduction + post-oxidation), was developed to completely remove nitrate and the long-term performance of nitrate reduction was comprehensively evaluated. The effluent nitrate steadily kept at 8.8 mg N/L during 120 h continuous operation when the influent nitrate concentration was 25.9 mg N/L. Simultaneously, nitrite concentration was lower than 0.01 mg N/L, and ammonium and Ti ions were not detected in the effluent.
中文翻译:

零价钛阳极间接电化学还原水中硝酸盐的影响因素,动力学和机理
在这项研究中,以零价钛(ZVT)为阳极的间接电化学还原成功地实现了从模拟地下水中选择性去除硝酸盐。12小时后,最大的硝酸盐去除效率和N 2选择性分别达到83.4%和78.5%。实验结果表明,气态副产物(NO和N 2 O)可以忽略不计,并且硝酸盐还原过程可以通过拟一级动力学模型很好地描述。降低电解质的pH值有利于电能的利用效率和硝酸盐的去除。氯化物最终显示出对硝酸盐电化学还原的抑制作用。在电化学反应期间,ZVT失去电子以生成还原剂(Ti 3+和Ti 2+),可以提供电子来还原硝酸盐并形成固体副产物TiO 2.4 Cl 0.2 N 0.1。为完全去除硝酸盐,开发了一种间接电化学还原+次氯酸盐处理(还原前+后氧化)两阶段策略,并对硝酸盐还原的长期性能进行了综合评估。当进水硝酸盐浓度为25.9 mg N / L时,在连续运行120小时的过程中,出水硝酸盐稳定地保持在8.8 mg N / L。同时,亚硝酸盐浓度低于0.01 mg N / L,并且在废水中未检测到铵离子和Ti离子。

































 京公网安备 11010802027423号
京公网安备 11010802027423号