Scientific Reports ( IF 3.8 ) Pub Date : 2019-03-29 , DOI: 10.1038/s41598-019-41800-2 Girijesh Kumar Patel , Mohammad Aslam Khan , Haseeb Zubair , Sanjeev Kumar Srivastava , Moh’d Khushman , Seema Singh , Ajay Pratap Singh
|
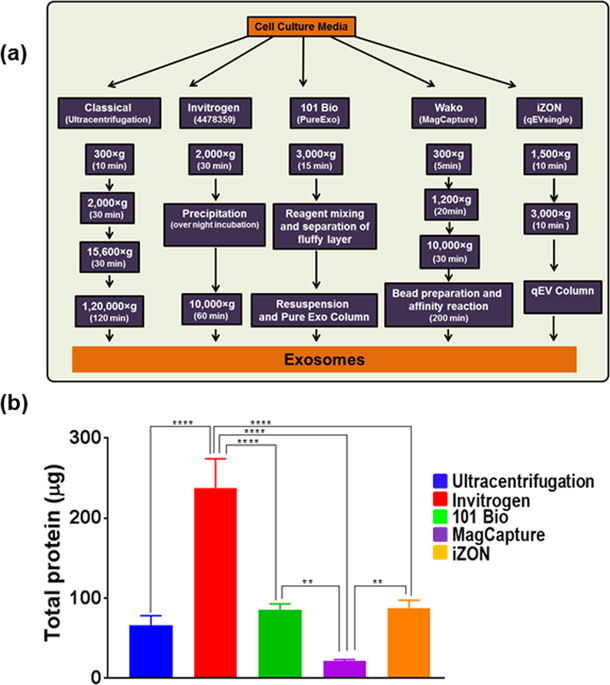
Exosomes have received significant attention for their role in pathobiological processes and are being explored as a tool for disease diagnosis and management. Consequently, various isolation methods based on different principles have been developed for exosome isolation. Here we compared the efficacy of four kits from Invitrogen, 101Bio, Wako and iZON along with conventional ultracentrifugation-based method for exosome yield, purity and quality. Cell culture supernatant was used as an abundant source of exosomes, and exosome quantity, size-distribution, zeta-potential, marker-expression and RNA/protein quality were determined. The Invitrogen kit gave the highest yield but the preparation showed broader size-distribution likely due to microvesicle co-precipitation and had the least dispersion stability. Other preparations showed <150 nm size range and good stability. Preparation from iZON column; however, had a broader size-distribution in the lower size range suggestive of some impurities of non-vesicular aggregates. RNA quality from all preparations was comparable; however, proteins from Invitrogen method-based exosomal preparation showed polyethylene glycol (PEG) contamination in mass spectrometry. Chemical impurities from the precipitant could also be the cause of toxicity of Invitrogen method-based exosomal preparation in biological growth measurement assay. Together, these findings should serve as a guide to choose and further optimize exosome isolation methods for their desired downstream applications.
中文翻译:

使用培养上清液进行外泌体分离方法的比较分析,以获得最佳产量,纯度和下游应用
外来体因其在病理生物学过程中的作用而受到广泛关注,并且正在被探索为疾病诊断和管理的工具。因此,已经开发了基于不同原理的各种分离方法用于外来体分离。在这里,我们比较了来自Invitrogen,101Bio,Wako和iZON的四种试剂盒以及基于超速离心的传统方法对外泌体产量,纯度和质量的功效。细胞培养上清液被用作外泌体的丰富来源,并测定了外泌体的数量,大小分布,ζ电位,标志物表达和RNA /蛋白质质量。Invitrogen试剂盒的收率最高,但制剂可能由于微泡共沉淀而显示出较宽的尺寸分布,并且分散稳定性最低。其他准备工作显示< 150 nm尺寸范围和良好的稳定性。从iZON柱进行制备;然而,在较低尺寸范围内具有较宽的尺寸分布,这表明非水泡聚集体中存在一些杂质。所有制备物中的RNA质量均具有可比性;但是,基于Invitrogen方法的外泌体制备物中的蛋白质在质谱中显示出聚乙二醇(PEG)污染。来自沉淀剂的化学杂质也可能是基于Invitrogen方法的外泌体制剂在生物生长测量分析中引起毒性的原因。总之,这些发现应作为指导,为他们所需的下游应用选择和进一步优化外泌体分离方法。在较低尺寸范围内具有较宽的尺寸分布,这表明存在非水泡聚集体的某些杂质。所有制备物中的RNA质量均具有可比性;但是,基于Invitrogen方法的外泌体制备物中的蛋白质在质谱中显示出聚乙二醇(PEG)污染。来自沉淀剂的化学杂质也可能是基于Invitrogen方法的外泌体制剂在生物生长测量分析中引起毒性的原因。总之,这些发现应作为指导,为他们所需的下游应用选择和进一步优化外泌体分离方法。在较低尺寸范围内具有较宽的尺寸分布,这表明存在非水泡聚集体的某些杂质。所有制备物中的RNA质量均具有可比性;但是,基于Invitrogen方法的外泌体制备物中的蛋白质在质谱中显示出聚乙二醇(PEG)污染。来自沉淀剂的化学杂质也可能是基于Invitrogen方法的外泌体制剂在生物生长测量分析中引起毒性的原因。总之,这些发现应作为指导,为他们所需的下游应用选择和进一步优化外泌体分离方法。沉淀剂中的化学杂质也可能是基于Invitrogen方法的外泌体制剂在生物生长测量分析中产生毒性的原因。总之,这些发现应作为指导,为他们所需的下游应用选择和进一步优化外泌体分离方法。来自沉淀剂的化学杂质也可能是基于Invitrogen方法的外泌体制剂在生物生长测量分析中引起毒性的原因。总之,这些发现应作为指导,为他们所需的下游应用选择和进一步优化外泌体分离方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号