当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Davis–Beirut Reaction: Alkoxide versus Hydroxide Addition to the Key o-Nitrosoimine Intermediate
Organic Letters ( IF 4.9 ) Pub Date : 2018-02-12 00:00:00 , DOI: 10.1021/acs.orglett.8b00036 Jie S Zhu 1 , Matthew R Duong 1 , Andrew P Teuthorn 1 , Julia Y Lu 1 , Jung-Ho Son 1 , Makhluf J Haddadin 2 , Mark J Kurth 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-02-12 00:00:00 , DOI: 10.1021/acs.orglett.8b00036 Jie S Zhu 1 , Matthew R Duong 1 , Andrew P Teuthorn 1 , Julia Y Lu 1 , Jung-Ho Son 1 , Makhluf J Haddadin 2 , Mark J Kurth 1
Affiliation
|
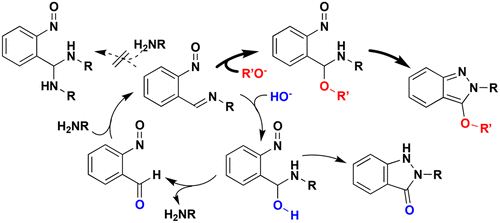
Reaction options, alkoxide vs hydroxide vs amine addition to the key intermediate (o-nitrosoimine) generated in the Davis–Beirut reaction of an o-nitrobenzylamine substrate, are reported to explain the nucleophilic addition selectivity of this one-pot indazole-forming process. The hydroxide addition/deprotection pathway as well as the fate of the resulting o-nitrosobenzaldehyde were both uncovered with several o-nitrobenzylamine substrates, and design elements required for an efficient double Davis–Beirut reaction, inspired by new mechanistic insights, were defined.
中文翻译:

戴维斯-贝鲁特反应:醇盐与氢氧化物加成到关键的邻亚硝基亚胺中间体
据报道,在邻硝基苯甲胺底物的戴维斯-贝鲁特反应中生成的关键中间体(邻亚硝基亚胺)上的醇盐、氢氧化物和胺加成反应选项,可以解释这一一锅吲唑形成过程的亲核加成选择性。氢氧化物加成/脱保护途径以及所得邻亚硝基苯甲醛的命运均通过几种邻硝基苯甲胺底物揭示,并在新的机理见解的启发下定义了高效双戴维斯-贝鲁特反应所需的设计元素。
更新日期:2018-02-12
中文翻译:

戴维斯-贝鲁特反应:醇盐与氢氧化物加成到关键的邻亚硝基亚胺中间体
据报道,在邻硝基苯甲胺底物的戴维斯-贝鲁特反应中生成的关键中间体(邻亚硝基亚胺)上的醇盐、氢氧化物和胺加成反应选项,可以解释这一一锅吲唑形成过程的亲核加成选择性。氢氧化物加成/脱保护途径以及所得邻亚硝基苯甲醛的命运均通过几种邻硝基苯甲胺底物揭示,并在新的机理见解的启发下定义了高效双戴维斯-贝鲁特反应所需的设计元素。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号