当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrite-Induced Activation of Iodate into Molecular Iodine in Frozen Solution
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2019-04-09 , DOI: 10.1021/acs.est.8b06638
Kitae Kim 1, 2 , Jinjung Ju 3 , Bomi Kim 1, 2 , Hyun Young Chung 1, 2 , L’ubica Vetráková 4 , Dominik Heger 4 , Alfonso Saiz-Lopez 5 , Wonyong Choi 6 , Jungwon Kim 3
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2019-04-09 , DOI: 10.1021/acs.est.8b06638
Kitae Kim 1, 2 , Jinjung Ju 3 , Bomi Kim 1, 2 , Hyun Young Chung 1, 2 , L’ubica Vetráková 4 , Dominik Heger 4 , Alfonso Saiz-Lopez 5 , Wonyong Choi 6 , Jungwon Kim 3
Affiliation
|
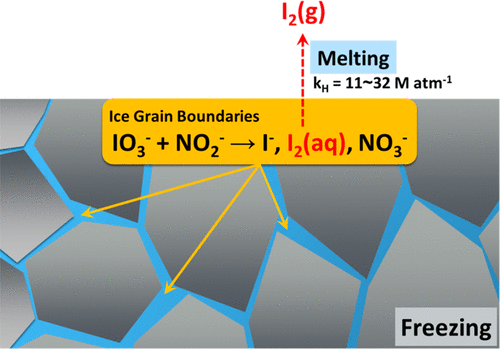
A new mechanism for the abiotic production of molecular iodine (I2) from iodate (IO3–), which is the most abundant iodine species, in dark conditions was identified and investigated. The production of I2 in aqueous solution containing IO3– and nitrite (NO2–) at 25 °C was negligible. However, the redox chemical reaction between IO3– and NO2– rapidly proceeded in frozen solution at −20 °C, which resulted in the production of I2, I–, and NO3–. The rapid redox chemical reaction between IO3– and NO2– in frozen solution is ascribed to the accumulation of IO3–, NO2–, and protons in the liquid regions between ice crystals during freezing (freeze concentration effect). This freeze concentration effect was verified by confocal Raman microscopy for the solute concentration and UV–visible absorption spectroscopy with cresol red (acid–base indicator) for the proton concentration. The freezing-induced production of I2 in the presence of IO3– and NO2– was observed under various conditions, which suggests this abiotic process for I2 production is not restricted to a specific region and occurs in many cold regions. NO2–-induced activation of IO3– to I2 in frozen solution may help explain why the measured values of iodine are larger than the modeled values in some polar areas.
中文翻译:

亚硝酸盐诱导的碘在冷冻溶液中活化为分子碘
鉴定并研究了在黑暗条件下从碘酸盐(IO 3 –)非生物生产分子碘(I 2)的新机制,碘酸盐是最丰富的碘物种。生产我2在含有IO水溶液3 -和亚硝酸盐(NO 2 - )在25℃下是微不足道的。然而,IO 3 –和NO 2 –之间的氧化还原化学反应在-20°C的冷冻溶液中迅速进行,导致产生I 2,I –和NO 3 –。IO 3之间的快速氧化还原化学反应–和NO 2 –在冷冻溶液中归因于IO 3 –,NO 2 –和质子在冷冻期间在冰晶之间的液体区域中的积累(冻结浓缩效应)。通过共聚焦拉曼显微镜对溶质浓度进行了验证,并使用了甲酚红(酸碱指示剂)对质子进行了紫外可见吸收光谱,验证了这种冻结浓缩效果。冷冻诱导的生产我2在IO的存在3 -和NO 2 -在各种条件下,观察到,这表明该非生物过程用于I 2生产并不局限于特定区域,而是发生在许多寒冷地区。NO 2 - IO的诱导激活3 -给我2冷冻液可能有助于解释为什么碘的测量值比一些极地地区的模拟值较大。
更新日期:2019-04-10
中文翻译:

亚硝酸盐诱导的碘在冷冻溶液中活化为分子碘
鉴定并研究了在黑暗条件下从碘酸盐(IO 3 –)非生物生产分子碘(I 2)的新机制,碘酸盐是最丰富的碘物种。生产我2在含有IO水溶液3 -和亚硝酸盐(NO 2 - )在25℃下是微不足道的。然而,IO 3 –和NO 2 –之间的氧化还原化学反应在-20°C的冷冻溶液中迅速进行,导致产生I 2,I –和NO 3 –。IO 3之间的快速氧化还原化学反应–和NO 2 –在冷冻溶液中归因于IO 3 –,NO 2 –和质子在冷冻期间在冰晶之间的液体区域中的积累(冻结浓缩效应)。通过共聚焦拉曼显微镜对溶质浓度进行了验证,并使用了甲酚红(酸碱指示剂)对质子进行了紫外可见吸收光谱,验证了这种冻结浓缩效果。冷冻诱导的生产我2在IO的存在3 -和NO 2 -在各种条件下,观察到,这表明该非生物过程用于I 2生产并不局限于特定区域,而是发生在许多寒冷地区。NO 2 - IO的诱导激活3 -给我2冷冻液可能有助于解释为什么碘的测量值比一些极地地区的模拟值较大。

































 京公网安备 11010802027423号
京公网安备 11010802027423号