当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of Iodinated Disinfection Byproducts (I-DBPs) in Drinking Water: Emerging Concerns and Current Issues
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-03-28 00:00:00 , DOI: 10.1021/acs.accounts.8b00641
Huiyu Dong 1, 2 , Zhimin Qiang 2 , Susan D. Richardson 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-03-28 00:00:00 , DOI: 10.1021/acs.accounts.8b00641
Huiyu Dong 1, 2 , Zhimin Qiang 2 , Susan D. Richardson 1
Affiliation
|
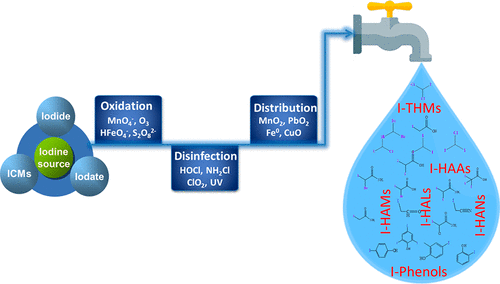
Formation of iodinated disinfection byproducts (I-DBPs) in drinking water has become an emerging concern. Compared to chlorine- and bromine-containing DBPs, I-DBPs are more toxic, have different precursors and formation mechanisms, and are unregulated. In this Account, we focus on recent research in the formation of known and unknown I-DBPs in drinking water. We present the state-of-the-art understanding of known I-DBPs for the six groups reported to date, including iodinated methanes, acids, acetamides, acetonitriles, acetaldehyde, and phenols. I-DBP concentrations in drinking water generally range from ng L–1 to low-μg L–1. The toxicological effects of I-DBPs are summarized and compared with those of chlorinated and brominated DBPs. I-DBPs are almost always more cytotoxic and genotoxic than their chlorinated and brominated analogues. Iodoacetic acid is the most genotoxic of all DBPs studied to date, and diiodoacetamide and iodoacetamide are the most cytotoxic. We discuss I-DBP formation mechanisms during oxidation, disinfection, and distribution of drinking water, focusing on inorganic and organic iodine sources, oxidation kinetics of iodide, and formation pathways. Naturally occurring iodide, iodate, and iodinated organic compounds are regarded as important sources of I-DBPs. The apparent second-order rate constant and half-lives for oxidation of iodide or hypoiodous acid by various oxidants are highly variable, which is a key factor governing the iodine fate during drinking water treatment. In distribution systems, residual iodide and disinfectants can participate in reactions involving heterogeneous chemical oxidation, reduction, adsorption, and catalysis, which may eventually affect I-DBP levels in finished drinking water. The identification of unknown I-DBPs and total organic iodine analysis is also summarized in this Account, which provides a more complete picture of I-DBP formation in drinking water. As organic DBP precursors are difficult to completely remove during the drinking water treatment process, the removal of iodide provides a cost-effective solution for the control of I-DBP formation. This Account not only serves as a reference for future epidemiological studies to better assess human health risks due to exposure to I-DBPs in drinking water but also helps drinking water utilities, researchers, regulators, and the general public understand the formed species, levels, and formation mechanisms of I-DBPs in drinking water.
中文翻译:

饮用水中碘化消毒副产物(I-DBPs)的形成:新出现的问题和当前的问题
饮用水中碘化消毒副产物(I-DBPs)的形成已成为一个新的关注点。与含氯和溴的DBP相比,I-DBP更具毒性,具有不同的前体和形成机理,并且不受管制。在此报告中,我们重点研究饮用水中已知和未知I-DBP形成的最新研究。我们介绍了迄今为止报道的六个组的已知I-DBP的最新知识,包括碘化甲烷,酸,乙酰胺,乙腈,乙醛和酚。饮用水中的I-DBP浓度范围通常从ng L –1到低μgL –1。总结了I-DBP的毒理作用,并将其与氯化和溴化DBP的毒理作用进行了比较。I-DBP几乎总是比其氯化和溴化类似物具有更高的细胞毒性和基因毒性。迄今为止,碘乙酸是所有DBP中最具遗传毒性的物质,二碘乙酰胺和碘乙酰胺是最具细胞毒性的物质。我们讨论了饮用水氧化,消毒和分配过程中的I-DBP形成机理,重点是无机和有机碘源,碘化物的氧化动力学以及形成途径。天然存在的碘化物,碘酸盐和碘化有机化合物被视为I-DBP的重要来源。各种氧化剂氧化碘或次碘酸的表观二阶速率常数和半衰期变化很大,这是控制饮用水处理过程中碘命运的关键因素。在分配系统中,残留的碘化物和消毒剂会参与涉及异质化学氧化,还原,吸附和催化的反应,这最终可能会影响成品饮用水中的I-DBP水平。该帐户还概述了未知I-DBP的鉴定和总有机碘分析,它提供了饮用水中I-DBP形成的更完整信息。由于在饮用水处理过程中很难完全去除有机DBP前体,因此碘化物的去除为控制I-DBP的形成提供了一种经济有效的解决方案。
更新日期:2019-03-28
中文翻译:

饮用水中碘化消毒副产物(I-DBPs)的形成:新出现的问题和当前的问题
饮用水中碘化消毒副产物(I-DBPs)的形成已成为一个新的关注点。与含氯和溴的DBP相比,I-DBP更具毒性,具有不同的前体和形成机理,并且不受管制。在此报告中,我们重点研究饮用水中已知和未知I-DBP形成的最新研究。我们介绍了迄今为止报道的六个组的已知I-DBP的最新知识,包括碘化甲烷,酸,乙酰胺,乙腈,乙醛和酚。饮用水中的I-DBP浓度范围通常从ng L –1到低μgL –1。总结了I-DBP的毒理作用,并将其与氯化和溴化DBP的毒理作用进行了比较。I-DBP几乎总是比其氯化和溴化类似物具有更高的细胞毒性和基因毒性。迄今为止,碘乙酸是所有DBP中最具遗传毒性的物质,二碘乙酰胺和碘乙酰胺是最具细胞毒性的物质。我们讨论了饮用水氧化,消毒和分配过程中的I-DBP形成机理,重点是无机和有机碘源,碘化物的氧化动力学以及形成途径。天然存在的碘化物,碘酸盐和碘化有机化合物被视为I-DBP的重要来源。各种氧化剂氧化碘或次碘酸的表观二阶速率常数和半衰期变化很大,这是控制饮用水处理过程中碘命运的关键因素。在分配系统中,残留的碘化物和消毒剂会参与涉及异质化学氧化,还原,吸附和催化的反应,这最终可能会影响成品饮用水中的I-DBP水平。该帐户还概述了未知I-DBP的鉴定和总有机碘分析,它提供了饮用水中I-DBP形成的更完整信息。由于在饮用水处理过程中很难完全去除有机DBP前体,因此碘化物的去除为控制I-DBP的形成提供了一种经济有效的解决方案。

































 京公网安备 11010802027423号
京公网安备 11010802027423号