当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemical Formation Process of Schwertmannite on Montmorillonite and Corresponding Cr(VI) Adsorption Capacity
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-03-27 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00202 Zhipeng Shu 1 , Lihu Liu 1 , Guohong Qiu 1 , Xiong Yang 1 , Mingzhe Zhang 1 , Wenfeng Tan 1 , Chengshuai Liu 2 , Fei Wu 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-03-27 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00202 Zhipeng Shu 1 , Lihu Liu 1 , Guohong Qiu 1 , Xiong Yang 1 , Mingzhe Zhang 1 , Wenfeng Tan 1 , Chengshuai Liu 2 , Fei Wu 2
Affiliation
|
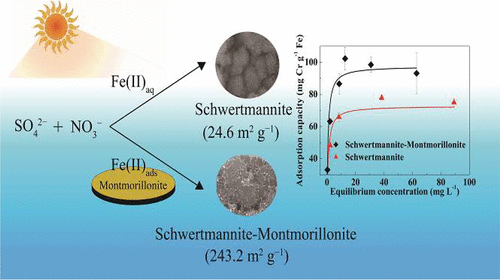
The formation and transformation of schwertmannite affect the migration, conversion, and toxicity of chromium (Cr) in soils and sediments. Schwertmannite could be obtained from the oxidation of Fe(II) by hydroxyl radicals (OH•) and superoxide radicals (O2•–) generated from the photolysis of NO3– under acidic and sulfate-rich conditions. As one of the most abundant components, montmorillonite is widely distributed in soils and sediments. However, the effect of montmorillonite on the photochemical formation process and corresponding Cr adsorption behaviors of schwertmannite remains elusive. This study indicates that schwertmannite could be formed on the montmorillonite surface during the photocatalytic oxidation of dissolved Fe(II). The formation rate, particle size, and crystallinity degree of schwertmannite formed on montmorillonite surface increased with increasing FeSO4 concentration (1.0–5.0 mmol L–1). The presence of montmorillonite led to a decrease in the particle size of schwertmannite. When the initial concentration of Fe(II) was 5.0 mmol L–1, the specific surface area of schwertmannite–montmorillonite aggregates reached 243.3 m2 g–1, which was remarkably larger than that of single-phase schwertmannite (24.6 m2 g–1) and montmorillonite (138.1 m2 g–1). The schwertmannite–montmorillonite aggregates showed a higher adsorption capacity for Cr(VI) (97.4 mg Cr g–1 Fe) than single-phase schwertmannite (72.9 mg Cr g–1 Fe). This work reveals the possible formation pathway and Cr adsorption behavior of schwertmannite on the surface of montmorillonite in waters and soils.
中文翻译:

Schwertmannite在蒙脱土上的光化学形成过程及相应的Cr(VI)吸附能力
schwertmannite的形成和转变会影响铬(Cr)在土壤和沉积物中的迁移,转化和毒性。可以通过羟基自由基(OH •)和超氧化物自由基(O 2 •–)氧化Fe(II)而获得的Schwertmannite,这些羟基自由基是由NO 3 –的光解产生的。在酸性和富含硫酸盐的条件下。蒙脱土作为最丰富的成分之一,广泛分布在土壤和沉积物中。然而,蒙脱石对光化学形成过程的影响以及schwertmannite的相应Cr吸附行为仍然难以捉摸。这项研究表明,在溶解的Fe(II)的光催化氧化过程中,schwertmannite可能形成在蒙脱石表面。随着FeSO 4浓度(1.0–5.0 mmol L –1)的增加,蒙脱石表面形成的schwertmannite的形成速率,粒径和结晶度增加。蒙脱石的存在导致schwertmannite粒度减小。当Fe(II)的初始浓度为5.0 mmol L –1时,Schwertmannite-蒙脱石聚集体的比表面积达到243.3 m 2 g –1,比单相Schwertmannite(24.6 m 2 g –1)和蒙脱石(138.1 m 2 g –1)的表面积大得多。schwertmannite-蒙脱土聚集体显示的对Cr(VI)(97.4 mg Cr g -1 Fe)的吸附能力高于单相schwertmannite(72.9 mg Cr g -1 Fe)的吸附能力。这项工作揭示了schwertmannite在水和土壤中的蒙脱石表面上可能的形成途径和Cr吸附行为。
更新日期:2019-03-27
中文翻译:

Schwertmannite在蒙脱土上的光化学形成过程及相应的Cr(VI)吸附能力
schwertmannite的形成和转变会影响铬(Cr)在土壤和沉积物中的迁移,转化和毒性。可以通过羟基自由基(OH •)和超氧化物自由基(O 2 •–)氧化Fe(II)而获得的Schwertmannite,这些羟基自由基是由NO 3 –的光解产生的。在酸性和富含硫酸盐的条件下。蒙脱土作为最丰富的成分之一,广泛分布在土壤和沉积物中。然而,蒙脱石对光化学形成过程的影响以及schwertmannite的相应Cr吸附行为仍然难以捉摸。这项研究表明,在溶解的Fe(II)的光催化氧化过程中,schwertmannite可能形成在蒙脱石表面。随着FeSO 4浓度(1.0–5.0 mmol L –1)的增加,蒙脱石表面形成的schwertmannite的形成速率,粒径和结晶度增加。蒙脱石的存在导致schwertmannite粒度减小。当Fe(II)的初始浓度为5.0 mmol L –1时,Schwertmannite-蒙脱石聚集体的比表面积达到243.3 m 2 g –1,比单相Schwertmannite(24.6 m 2 g –1)和蒙脱石(138.1 m 2 g –1)的表面积大得多。schwertmannite-蒙脱土聚集体显示的对Cr(VI)(97.4 mg Cr g -1 Fe)的吸附能力高于单相schwertmannite(72.9 mg Cr g -1 Fe)的吸附能力。这项工作揭示了schwertmannite在水和土壤中的蒙脱石表面上可能的形成途径和Cr吸附行为。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号