当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of Axially Chiral 2,2′-Biimidazole Ligands through Remote Chirality Delivery and Their Application in Asymmetric Carbene Insertion into N–H of Carbazoles
Organic Letters ( IF 4.9 ) Pub Date : 2019-03-28 00:00:00 , DOI: 10.1021/acs.orglett.9b00687 Hong-Qiang Shen 1, 2 , Bo Wu 1 , Huan-Ping Xie 1 , Yong-Gui Zhou 1, 3
Organic Letters ( IF 4.9 ) Pub Date : 2019-03-28 00:00:00 , DOI: 10.1021/acs.orglett.9b00687 Hong-Qiang Shen 1, 2 , Bo Wu 1 , Huan-Ping Xie 1 , Yong-Gui Zhou 1, 3
Affiliation
|
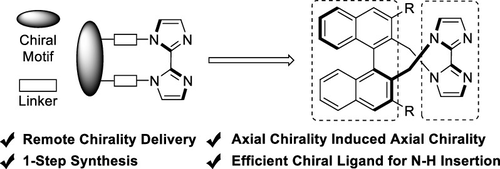
Axially chiral biimidazole ligands have been rarely synthesized and studied, in contrast to the significant achievements in the synthesis and application of central chiral imidazole ligands. Herein, a series of novel axially chiral 2,2′-biimidazole ligands were synthesized from the reaction of 2,2′-bis(bromomethyl)-1,1′-binaphthalene and 2,2′-biimidazole in one step through the strategy of remote chirality delivery. These ligands have been proven to be efficient for Cu- or Fe-catalyzed asymmetric insertion of α-aryl-α-diazoacetates into the N–H bond of carbazoles with up to 96% ee.
中文翻译:

远程手性传递法制备轴向手性2,2'-苯并咪唑配体及其在咔唑不对称碳原子插入NH中的应用
与在中心手性咪唑配体的合成和应用方面取得的显著成就相反,轴向手性联咪唑配体很少被合成和研究。在此,通过该策略一步一步地由2,2'-双(溴甲基)-1,1'-联萘与2,2'-联咪唑的反应合成了一系列新型的轴向手性2,2'-联咪唑配体手性交付。这些配体已被证明可有效地将Cu-或Fe催化的α-芳基-α-重氮乙酸酯的不对称插入ee高达96%的咔唑的N–H键中。
更新日期:2019-03-28
中文翻译:

远程手性传递法制备轴向手性2,2'-苯并咪唑配体及其在咔唑不对称碳原子插入NH中的应用
与在中心手性咪唑配体的合成和应用方面取得的显著成就相反,轴向手性联咪唑配体很少被合成和研究。在此,通过该策略一步一步地由2,2'-双(溴甲基)-1,1'-联萘与2,2'-联咪唑的反应合成了一系列新型的轴向手性2,2'-联咪唑配体手性交付。这些配体已被证明可有效地将Cu-或Fe催化的α-芳基-α-重氮乙酸酯的不对称插入ee高达96%的咔唑的N–H键中。





























 京公网安备 11010802027423号
京公网安备 11010802027423号