European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-03-26 , DOI: 10.1016/j.ejmech.2019.03.055 Guanghui Tang , Lihong Liu , Xueying Wang , Zhengying Pan
|
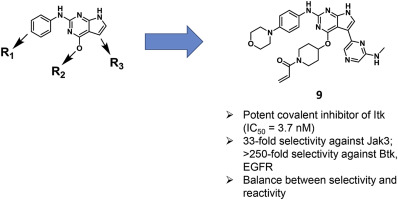
Interleukin-2-inducible T-cell kinase (Itk) plays an important role in multiple signal transduction pathways in T and mast cells, and is a potential drug target for treating inflammatory diseases, autoimmune diseases, and T cell leukemia/lymphoma. Herein, we describe the discovery of a series of covalent Itk inhibitors based on the 7H-pyrrolo[2,3-d]pyrimidine scaffold. Placing an appropriate substitution group at a hydration site of the ATP binding pocket of Itk and using a saturated heterocyclic ring as a linker to the reactive group were crucial for selectivity. The optimized compound 9 showed potent activity against Itk, excellent selectivity for Itk over Btk and other structurally related kinases, inhibition of phospholipase C-γ1 (PLC-γ1) phosphorylation in cells, and anti-proliferative effects against multiple T leukemia/lymphoma cell lines. Compound 9 can serve as a valuable compound for further determination of functions of Itk.
中文翻译:

发现7 H-吡咯并[2,3- d ]嘧啶衍生物作为白介素2诱导性T细胞激酶(Itk)的选择性共价不可逆抑制剂
白介素2诱导型T细胞激酶(Itk)在T和肥大细胞的多种信号转导途径中起着重要作用,并且是治疗炎性疾病,自身免疫性疾病和T细胞白血病/淋巴瘤的潜在药物靶标。在这里,我们描述了基于7 H-吡咯并[2,3- d ]嘧啶骨架的一系列共价Itk抑制剂的发现。在Itk的ATP结合口袋的水合位点处放置一个适当的取代基,并使用饱和的杂环作为反应基团的连接剂,对选择性至关重要。优化的化合物9表现出对Itk的有效活性,对Itk的优于Btk和其他结构相关激酶的选择性,对细胞中磷脂酶C-γ1(PLC-γ1)磷酸化的抑制作用以及对多种T白血病/淋巴瘤细胞系的抗增殖作用。化合物9可用作进一步确定Itk功能的有价值的化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号