当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalization of Enzymatically Synthesized Rigid Poly(itaconate)s via Post‐Polymerization Aza‐Michael Addition of Primary Amines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-04-12 , DOI: 10.1002/adsc.201900055 Alice Guarneri 1, 2 , Viola Cutifani 1 , Marco Cespugli 1 , Alessandro Pellis 1, 3 , Roberta Vassallo 1 , Fioretta Asaro 1 , Cynthia Ebert 1 , Lucia Gardossi 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-04-12 , DOI: 10.1002/adsc.201900055 Alice Guarneri 1, 2 , Viola Cutifani 1 , Marco Cespugli 1 , Alessandro Pellis 1, 3 , Roberta Vassallo 1 , Fioretta Asaro 1 , Cynthia Ebert 1 , Lucia Gardossi 1
Affiliation
|
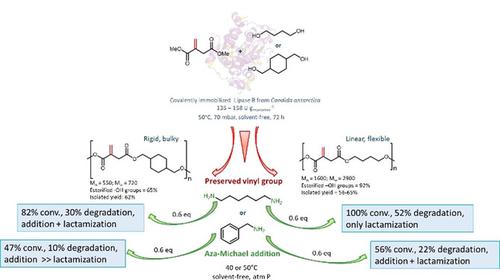
The bulky 1,4‐cyclohexanedimethanol was used as co‐monomer for introducing rigidity in lipase synthetized poly(itaconate)s. Poly(1,4‐cyclohexanedimethanol itaconate) was synthetized on a 14 g scale at 50 °C, under solvent‐free conditions and 70 mbar using only 135 Units of lipase B from Candida antarctica per gram of monomer. The mild conditions preserved the labile vinyl group of itaconic acid and avoided the decomposition of 1,4‐cyclohexanedimethanol, both observed in chemical polycondensations. Experimental and computational data show that the enzymatic polycondensation proceeds despite the low reactivity of C1 of itaconic acid. The rigid poly(1,4‐cyclohexanedimethanol itaconate) was investigated in the context of aza‐Michael addition of hexamethylenediamine and 2‐phenylethylamine to the vinyl moiety. The enzymatically synthesized linear poly(1,4‐butylene itaconate) was studied as a comparison. The two oligoesters (Molecular Weights ranging from 720 to 2859 g mol−1) reacted on a gram scale, at 40–50 °C, at atmospheric pressure and in solvent‐free conditions. The addition of primary amines led to amine‐functionalized oligoesters but also to chain degradation, and the reactivity of the poly(itaconate)s was influenced by the rigidity of the polymer chain. Upon the formation of the secondary amine adduct, the linear poly(1,4‐butylene itaconate) undergoes fast intramolecular cyclization and subsequent degradation via pyrrolidone formation, especially in the presence of hexamethylenediamine. On the contrary, the bulky 1,4‐cyclohexanedimethanol confers rigidity to poly(1,4‐cyclohexanedimethanol itaconate), which hampers the intramolecular cyclization. Also, the bulkiness of the amine and the use of solvent emerged as factors that affect the reactivity of poly(itaconate)s. Therefore, the possibility to insert discrete units of itaconic acid in oligoesters using biocatalysts under solvent‐free mild conditions opens new routes for the generation of bio‐based functional polymers or amine‐triggered degradable materials, as a function of the rigidity of the polyester chain.
中文翻译:

通过伯胺的聚合反应后的Aza-Michael加成功能实现酶促合成的刚性聚衣康酸酯的功能化
庞大的1,4-环己烷二甲醇被用作共聚单体,以在脂肪酶合成的聚衣康酸酯中引入刚性。仅在每克单体中使用135单位南极假丝酵母的脂肪酶B,在无溶剂条件下和70 mbar下于50°C于14 g规模合成聚(1,4-环己烷二甲醇衣康酸酯)。温和的条件保留了衣康酸的不稳定乙烯基,避免了1,4-环己烷二甲醇的分解,这在化学缩聚反应中都可以观察到。实验和计算数据表明,尽管C 1的反应性较低,但酶促缩聚仍在进行衣康酸。刚性聚(1,4-环己烷二甲醇衣康酸酯)是在六亚甲基二胺和2-苯基乙胺的氮杂-迈克尔加成至乙烯基部分的背景下进行研究的。作为比较,研究了酶促合成的线性聚(1,4-丁烯衣康酸酯)。两种低聚酯(分子量范围为720至2859 g mol -1)在40–50°C,大气压和无溶剂条件下以克为单位反应。伯胺的加入导致胺官能化的低聚酯,但也导致链降解,聚衣康酸酯的反应性受到聚合物链刚性的影响。形成仲胺加合物后,线性聚(1,4-丁烯衣康酸酯)会快速发生分子内环化反应,并随后通过吡咯烷酮形成而降解,特别是在六亚甲基二胺存在下。相反,笨重的1,4-环己烷二甲醇衣康酸酯赋予聚(1,4-环己烷二甲醇衣康酸酯)刚性,这阻碍了分子内环化。同样,胺的体积大和溶剂的使用也成为影响聚衣康酸酯的反应性的因素。所以,
更新日期:2019-04-12
中文翻译:

通过伯胺的聚合反应后的Aza-Michael加成功能实现酶促合成的刚性聚衣康酸酯的功能化
庞大的1,4-环己烷二甲醇被用作共聚单体,以在脂肪酶合成的聚衣康酸酯中引入刚性。仅在每克单体中使用135单位南极假丝酵母的脂肪酶B,在无溶剂条件下和70 mbar下于50°C于14 g规模合成聚(1,4-环己烷二甲醇衣康酸酯)。温和的条件保留了衣康酸的不稳定乙烯基,避免了1,4-环己烷二甲醇的分解,这在化学缩聚反应中都可以观察到。实验和计算数据表明,尽管C 1的反应性较低,但酶促缩聚仍在进行衣康酸。刚性聚(1,4-环己烷二甲醇衣康酸酯)是在六亚甲基二胺和2-苯基乙胺的氮杂-迈克尔加成至乙烯基部分的背景下进行研究的。作为比较,研究了酶促合成的线性聚(1,4-丁烯衣康酸酯)。两种低聚酯(分子量范围为720至2859 g mol -1)在40–50°C,大气压和无溶剂条件下以克为单位反应。伯胺的加入导致胺官能化的低聚酯,但也导致链降解,聚衣康酸酯的反应性受到聚合物链刚性的影响。形成仲胺加合物后,线性聚(1,4-丁烯衣康酸酯)会快速发生分子内环化反应,并随后通过吡咯烷酮形成而降解,特别是在六亚甲基二胺存在下。相反,笨重的1,4-环己烷二甲醇衣康酸酯赋予聚(1,4-环己烷二甲醇衣康酸酯)刚性,这阻碍了分子内环化。同样,胺的体积大和溶剂的使用也成为影响聚衣康酸酯的反应性的因素。所以,































 京公网安备 11010802027423号
京公网安备 11010802027423号