当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogenation of (Hetero)aryl Boronate Esters with a Cyclic (Alkyl)(amino)carbene–Rhodium Complex: Direct Access to cis‐Substituted Borylated Cycloalkanes and Saturated Heterocycles
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-04-09 , DOI: 10.1002/anie.201811210 Liang Ling 1 , Yuan He 1 , Xue Zhang 1 , Meiming Luo 1 , Xiaoming Zeng 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-04-09 , DOI: 10.1002/anie.201811210 Liang Ling 1 , Yuan He 1 , Xue Zhang 1 , Meiming Luo 1 , Xiaoming Zeng 1
Affiliation
|
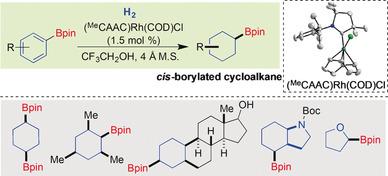
We herein report the hydrogenation of substituted aryl‐ and heteroaryl boronate esters for the selective synthesis of cis‐substituted borylated cycloalkanes and saturated heterocycles. A cyclic (alkyl)(amino)carbene‐ligated rhodium complex with two dimethyl groups at the ortho‐alkyl scaffold of the carbene showed high reactivity in promoting the hydrogenation, thereby enabling the hydrogenation of (hetero)arenes with retention of the synthetically valuable boronate group. This process constitutes a clean, atom‐economic, as well as chemo‐ and stereoselective route for the generation of cis‐configured, diversely substituted borylated cycloalkanes and saturated heterocycles that are usually elusive and difficult to prepare.
中文翻译:

用环状(烷基)(氨基)碳烯-铑配合物氢化(杂)硼酸芳基酯:直接获得顺式取代的硼化环烷烃和饱和杂环
我们在此报告了取代的芳基和杂芳基硼酸酯的氢化反应,用于选择性合成顺式取代的硼化环烷烃和饱和杂环。在卡宾的邻烷基支架上带有两个二甲基的环状(烷基)(氨基)卡宾连接的铑配合物在促进氢化方面显示出高反应活性,从而使(杂)芳烃得以氢化并保留了合成有价值的硼酸酯团体。该过程构成了清洁,原子经济以及化学和立体选择性的途径,用于生成通常难以捉摸且难以制备的顺式构型,不同取代的硼酸化环烷烃和饱和杂环。
更新日期:2019-04-09
中文翻译:

用环状(烷基)(氨基)碳烯-铑配合物氢化(杂)硼酸芳基酯:直接获得顺式取代的硼化环烷烃和饱和杂环
我们在此报告了取代的芳基和杂芳基硼酸酯的氢化反应,用于选择性合成顺式取代的硼化环烷烃和饱和杂环。在卡宾的邻烷基支架上带有两个二甲基的环状(烷基)(氨基)卡宾连接的铑配合物在促进氢化方面显示出高反应活性,从而使(杂)芳烃得以氢化并保留了合成有价值的硼酸酯团体。该过程构成了清洁,原子经济以及化学和立体选择性的途径,用于生成通常难以捉摸且难以制备的顺式构型,不同取代的硼酸化环烷烃和饱和杂环。































 京公网安备 11010802027423号
京公网安备 11010802027423号