当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stabilization of Formate Dehydrogenase in a Metal-Organic Framework for Bioelectrocatalytic Reduction of CO2.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-05-02 , DOI: 10.1002/anie.201901981 Yijing Chen,Peng Li,Hyunho Noh,Chung-Wei Kung,Cassandra T Buru,Xingjie Wang,Xuan Zhang,Omar K Farha
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-05-02 , DOI: 10.1002/anie.201901981 Yijing Chen,Peng Li,Hyunho Noh,Chung-Wei Kung,Cassandra T Buru,Xingjie Wang,Xuan Zhang,Omar K Farha
|
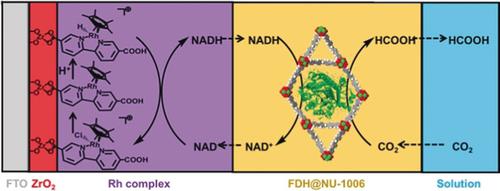
The efficient fixation of excess CO2 from the atmosphere to yield value‐added chemicals remains crucial in response to the increasing levels of carbon emission. Coupling enzymatic reactions with electrochemical regeneration of cofactors is a promising technique for fixing CO2, while producing biomass which can be further transformed into biofuels. Herein, a bioelectrocatalytic system was established by depositing crystallites of a mesoporous metal–organic framework (MOF), termed NU‐1006, containing formate dehydrogenase, on a fluorine‐doped tin oxide glass electrode modified with Cp*Rh(2,2′‐bipyridyl‐5,5′‐dicarboxylic acid)Cl2 complex. This system converts CO2 into formic acid at a rate of 79±3.4 mm h−1 with electrochemical regeneration of the nicotinamide adenine dinucleotide cofactor. The MOF–enzyme composite exhibited significantly higher catalyst stability when subjected to non‐native conditions compared to the free enzyme, doubling the formic acid yield.
中文翻译:

金属有机框架中甲酸甲脱氢酶的稳定性用于生物电催化还原CO2。
应对不断增加的碳排放水平,有效地将大气中过量的CO 2固定为增产值的化学品仍然至关重要。酶促反应与辅因子的电化学再生耦合是固定CO 2的有前途的技术,同时产生可进一步转化为生物燃料的生物质。在此,通过在含有Cp * Rh(2,2'联吡啶-5,5'-二羧酸)Cl 2络合物 该系统以79±3.4 m m h的速率将CO 2转化为甲酸-1具有烟酰胺腺嘌呤二核苷酸辅因子的电化学再生。与游离酶相比,MOF-酶复合物在非天然条件下表现出明显更高的催化剂稳定性,使甲酸收率提高了一倍。
更新日期:2019-05-02
中文翻译:

金属有机框架中甲酸甲脱氢酶的稳定性用于生物电催化还原CO2。
应对不断增加的碳排放水平,有效地将大气中过量的CO 2固定为增产值的化学品仍然至关重要。酶促反应与辅因子的电化学再生耦合是固定CO 2的有前途的技术,同时产生可进一步转化为生物燃料的生物质。在此,通过在含有Cp * Rh(2,2'联吡啶-5,5'-二羧酸)Cl 2络合物 该系统以79±3.4 m m h的速率将CO 2转化为甲酸-1具有烟酰胺腺嘌呤二核苷酸辅因子的电化学再生。与游离酶相比,MOF-酶复合物在非天然条件下表现出明显更高的催化剂稳定性,使甲酸收率提高了一倍。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号