Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2019-03-27 , DOI: 10.1016/j.cej.2019.03.237 Yu-qiong Gao , Nai-yun Gao , Wen-hai Chu , Ya-feng Zhang , Jia Zhang , Da-qiang Yin
|
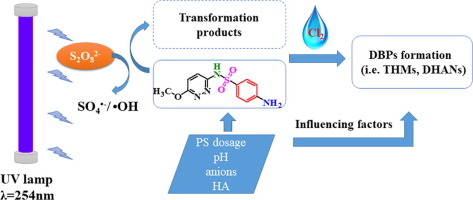
The kinetics and mechanisms of the degradation of sulfamethoxypyridazine (SMP) by an ultraviolet-activated persulfate (UV/PS) advanced oxidation process (AOP) and the formation of disinfection byproducts (DBPs) during subsequent chlorination were investigated in this study. The UV/PS process can significantly remove SMP through pseudo-first-order reaction kinetics. In a competitive kinetic experiment, the second-order rate constant of SMP with sulfate radical was determined to be 2.73 × 1010 M−1 s−1, while that with hydroxyl radical was 2.22 × 1010 M−1 s−1. Six major transformation products in SMP degradation were recognized by LC/MS/MS analysis. It was assumed that the degradation pathway of SMP involves the hydroxylation of the aromatic ring, cleavage of the sulfonamide bond, oxidation of the aniline moiety and elimination of SO2. The impacts of persulfate dose, pH, anions (HCO3–, SO42−, and Br−) and humic acid (HA) concentration on SMP degradation efficiency and DBP formation during subsequent chlorination were also examined. SMP degradation was accelerated with increasing persulfate dose, HCO3– and Br- concentration as well as decreasing pH and HA concentration. However, the amount of chloroform (CF) formed was reduced under a higher persulfate dosage and lower pH. In contrast, the amount of dichloroacetonitrile (DCAN) formed was enhanced under a higher persulfate dose and lower pH. Adding HA also increased the formation of both CF and DCAN. While, both HCO3– and SO42– had little effects on the formation of CF and DCAN. The presence of Br– had no significant effects on the bromine incorporation factors (BIFs) of trihalomethanes (THMs) and dihaloacetonitriles (DHANs). Raw water experiments showed that the UV/PS process could destroy SMP in natural water as well as control the formation of DBPs.
中文翻译:

磺胺甲氧哒嗪的紫外线活化过硫酸盐氧化:动力学,降解途径及其对随后氯化反应中DBP形成的影响
在本研究中,研究了通过紫外线活化的过硫酸盐(UV / PS)高级氧化过程(AOP)降解磺胺甲氧基哒嗪(SMP)的动力学和机理,以及后续氯化过程中消毒副产物(DBPs)的形成。UV / PS工艺可以通过拟一级反应动力学显着去除SMP。在竞争动力学实验中,硫酸根自由基的SMP二级速率常数确定为2.73×10 10 M -1 s -1,而羟基自由基的SMP的二级速率常数为2.22×10 10 M -1 s -1。LC / MS / MS分析识别出SMP降解的六种主要转化产物。据推测,SMP的降解途径涉及芳环的羟基化,磺酰胺键的裂解,苯胺部分的氧化和SO 2的消除。过剂量,pH值,阴离子的影响(HCO 3 -,SO 4 2-,和Br -还检查了在SMP降解效率和DBP的形成随后的氯化过程中和腐殖酸(HA)浓度)。SMP降解随过剂量,HCO加速3 -和Br -浓度以及降低pH和HA浓度。但是,在较高的过硫酸盐剂量和较低的pH值下,形成的氯仿(CF)的量减少了。相反,在较高的过硫酸盐剂量和较低的pH值下,形成的二氯乙腈(DCAN)的量增加了。添加HA还增加了CF和DCAN的形成。而,无论是HCO 3 -和SO 4 2-对CF和DCAN的形成影响很小。BR的存在-对三卤甲烷(三卤甲烷)和dihaloacetonitriles(DHANs)的溴结合因子(的BIF)不显著影响。原水实验表明,UV / PS工艺可以破坏天然水中的SMP并控制DBP的形成。































 京公网安备 11010802027423号
京公网安备 11010802027423号