Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2019-03-25 , DOI: 10.1038/s41594-019-0204-3 Yu Pang 1 , Hayashi Yamamoto 2 , Hirokazu Sakamoto 2 , Masahide Oku 3 , Joe Kimanthi Mutungi 2, 4 , Mayurbhai Himatbhai Sahani 2, 5 , Yoshitaka Kurikawa 2 , Kiyoshi Kita 6, 7, 8 , Nobuo N Noda 9 , Yasuyoshi Sakai 3 , Honglin Jia 1 , Noboru Mizushima 2
|
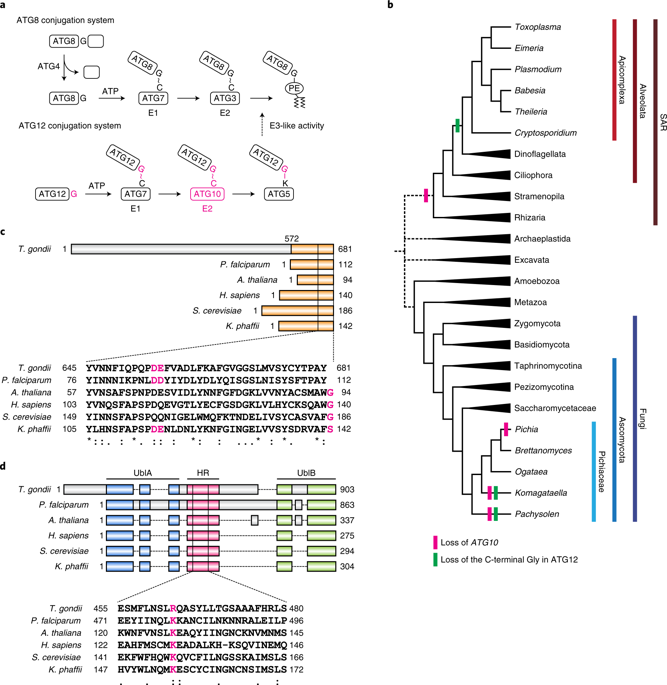
Ubiquitin or ubiquitin-like proteins can be covalently conjugated to multiple proteins that do not necessarily have binding interfaces. Here, we show that an evolutionary transition from covalent conjugation to non-covalent interaction has occurred in the ubiquitin-like autophagy-related 12 (ATG12) conjugation system. ATG12 is covalently conjugated to its sole substrate, ATG5, by a ubiquitylation-like mechanism. However, the apicomplexan parasites Plasmodium and Toxoplasma and some yeast species such as Komagataella phaffii (previously Pichia pastoris) lack the E2-like enzyme ATG10 and the most carboxy (C)-terminal glycine of ATG12, both of which are required for covalent linkage. Instead, ATG12 in these organisms forms a non-covalent complex with ATG5. This non-covalent ATG12–ATG5 complex retains the ability to facilitate ATG8–phosphatidylethanolamine conjugation. These results suggest that ubiquitin-like covalent conjugation can evolve to a simpler non-covalent interaction, most probably when the system has a limited number of targets.
中文翻译:

遍在蛋白样ATG12系统中从共价结合演变为非共价相互作用
遍在蛋白或类遍在蛋白可以共价结合到不一定具有结合界面的多种蛋白上。在这里,我们表明泛素样自噬相关的12(ATG12)缀合系统中发生了从共价缀合到非共价相互作用的进化过渡。ATG12通过类泛素化机制共价缀合至其唯一底物ATG5。然而,apicomplexan寄生虫疟原虫和弓形虫和一些酵母菌,例如Komagataella phaffii(以前称为毕赤酵母))缺乏类似E2的酶ATG10和ATG12的羧基(C)末端最多的甘氨酸,这两者都是共价连接所必需的。相反,这些生物中的ATG12与ATG5形成非共价复合物。这种非共价的ATG12–ATG5复合物保留了促进ATG8–磷脂酰乙醇胺结合的能力。这些结果表明,泛素样共价结合可以演变为更简单的非共价相互作用,最可能是在系统具有有限数量的靶标的情况下。































 京公网安备 11010802027423号
京公网安备 11010802027423号