Nature Energy ( IF 49.7 ) Pub Date : 2019-03-25 , DOI: 10.1038/s41560-019-0355-9 Zhen-Feng Huang , Jiajia Song , Yonghua Du , Shibo Xi , Shuo Dou , Jean Marie Vianney Nsanzimana , Cheng Wang , Zhichuan J. Xu , Xin Wang
|
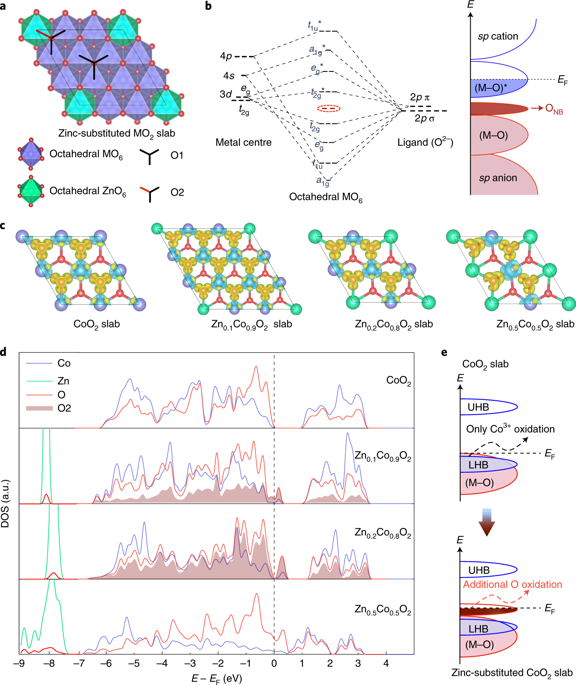
The oxygen evolution reaction (OER) is a key process in electrochemical energy conversion devices. Understanding the origins of the lattice oxygen oxidation mechanism is crucial because OER catalysts operating via this mechanism could bypass certain limitations associated with those operating by the conventional adsorbate evolution mechanism. Transition metal oxyhydroxides are often considered to be the real catalytic species in a variety of OER catalysts and their low-dimensional layered structures readily allow direct formation of the O–O bond. Here, we incorporate catalytically inactive Zn2+ into CoOOH and suggest that the OER mechanism is dependent on the amount of Zn2+ in the catalyst. The inclusion of the Zn2+ ions gives rise to oxygen non-bonding states with different local configurations that depend on the quantity of Zn2+. We propose that the OER proceeds via the lattice oxygen oxidation mechanism pathway on the metal oxyhydroxides only if two neighbouring oxidized oxygens can hybridize their oxygen holes without sacrificing metal–oxygen hybridization significantly, finding that Zn0.2Co0.8OOH has the optimum activity.
中文翻译:

Co-Zn羟基氧化析氧电催化剂中晶格氧氧化的化学和结构起源
氧气释放反应(OER)是电化学能量转换设备中的关键过程。理解晶格氧氧化机理的起源是至关重要的,因为通过该机理操作的OER催化剂可以绕过与常规吸附物逸出机理操作相关的某些限制。过渡金属羟基氧化物通常被认为是各种OER催化剂中的真正催化物质,它们的低维分层结构易于直接形成O-O键。在这里,我们将无催化活性的Zn 2+引入CoOOH中,并表明OER机理取决于催化剂中Zn 2+的量。包含Zn 2+离子会根据Zn 2+的数量而产生具有不同局部构型的氧非键合态。我们提出,只有在两个相邻的氧化氧可以在不显着牺牲金属-氧杂化的情况下使它们的氧孔杂交,OER才通过金属羟基氧化物上的晶格氧氧化机理途径进行,发现Zn 0.2 Co 0.8 OOH具有最佳活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号