Nature Catalysis ( IF 42.8 ) Pub Date : 2019-03-25 , DOI: 10.1038/s41929-019-0247-1
Liang-Wen Qi , Shaoyu Li , Shao-Hua Xiang , Jun Wang , Bin Tan
|
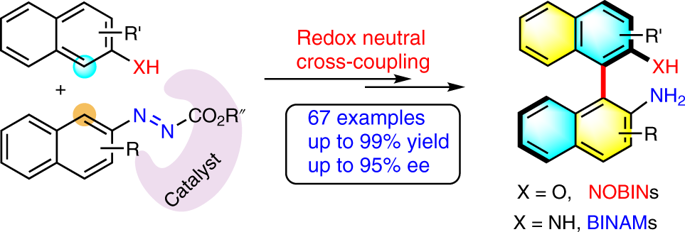
Atropisomerically enriched biaryl frameworks are ubiquitous in many fields of chemistry. Enantioselective aryl–aryl cross-coupling provides the most straightforward entry to atropisomeric biaryls, with remarkable application potential in the field of chemical science. However, their development is hindered due to the lack of convenient and pragmatic protocols. Here, we report a method for the asymmetric synthesis of a myriad of 2-amino-2′-hydroxy-1,1′-binaphthyl (NOBIN) and 1,1’-binaphthyl-2,2’-diamine (BINAM) derivatives in excellent yields and enantioselectivities via a redox-neutral cross-coupling protocol. Two complementary systems were devised employing a chiral phosphoric acid–salt complex or Ni(OTf)2/chiral bis(oxazoline) ligand catalytic system for accessing atropisomeric NOBIN and BINAM derivatives, respectively. This work provides an alternative avenue to enantioenriched biaryls, and provides the capability to explore the synthetic and catalytic potentials of NOBIN- and BINAM-based frameworks.
中文翻译:

通过氧化还原中性交叉偶联策略不对称构造阻转异构体联芳基
阻气异构的联芳构架在许多化学领域中普遍存在。对映选择性芳基-芳基交叉偶联提供了最简单的阻转异构联芳基化合物,在化学科学领域具有巨大的应用潜力。但是,由于缺乏方便实用的协议,阻碍了它们的发展。在这里,我们报告了一种不对称合成大量的2-氨基-2'-羟基-1,1'-联萘(NOBIN)和1,1'-联萘-2,2'-二胺(BINAM)衍生物的方法通过氧化还原中性交叉偶联方案获得优异的收率和对映选择性。使用手性磷酸-盐络合物或Ni(OTf)2设计了两个互补系统/手性双(恶唑啉)配体催化体系,分别用于获得阻转异构的NOBIN和BINAM衍生物。这项工作提供了对映体丰富的联芳烃的替代途径,并提供了探索基于NOBIN和BINAM的框架的合成和催化潜力的能力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号