当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Orthophosphate and Sulfate Utilization for C–E (E = P, S) Bond Formation via Trichlorosilyl Phosphide and Sulfide Anions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-03-22 , DOI: 10.1021/jacs.9b01475 Michael B Geeson 1 , Pablo Ríos 1 , Wesley J Transue 1 , Christopher C Cummins 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-03-22 , DOI: 10.1021/jacs.9b01475 Michael B Geeson 1 , Pablo Ríos 1 , Wesley J Transue 1 , Christopher C Cummins 1
Affiliation
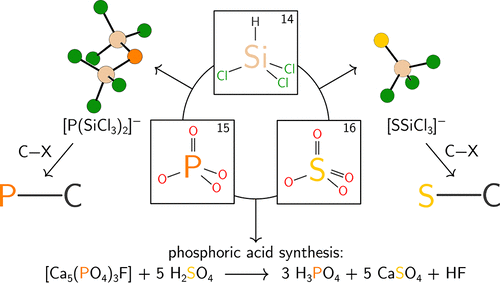
|
Reduction of phosphoric acid (H3PO4) or tetra- n-butylammonium bisulfate ([TBA][HSO4]) with trichlorosilane leads to the formation of the bis(trichlorosilyl)phosphide ([P(SiCl3)2]-, 1) and trichlorosilylsulfide ([Cl3SiS]-, 2) anions, respectively. Balanced equations for the formation of the TBA salts of anions 1 and 2 were formulated based on the identification of hexachlorodisiloxane and hydrogen gas as byproducts arising from these reductive processes: i) [H2PO4]- + 10HSiCl3 → 1 + 4O(SiCl3)2 + 6H2 for P and ii) [HSO4]- + 9HSiCl3 → 2 + 4O(SiCl3)2 + 5H2 for S. Hydrogen gas was identified by its subsequent use to hydrogenate an alkene ((-)-terpinen-4-ol) using Crabtree's catalyst ([(COD)Ir(py)(PCy3)][PF6], COD = 1,5-cyclooctadiene, py = pyridine, Cy = cyclohexyl). Phosphide 1 was generated in situ by the reaction of phosphoric acid and trichlorosilane and used to convert an alkyl chloride (1-chlorooctane) to the corresponding primary phosphine, which was isolated in 41% yield. Anion 1 was also prepared from [TBA][H2PO4] and isolated in 62% yield on a gram scale. Treatment of [TBA]1 with an excess of benzyl chloride leads to the formation of tetrabenzylphosphonium chloride, which was isolated in 61% yield. Sulfide 2 was used as a thionation reagent, converting benzophenone to thiobenzophenone in 62% yield. It also converted benzyl bromide to benzyl mercaptan in 55% yield. The TBA salt of trimetaphosphate ([TBA]3[P3O9]·2H2O), also a precursor to anion 1, was found to react with either trichlorosilane or silicon(IV) chloride to provide bis(trimetaphosphate)silicate, [TBA]2[Si(P3O9)2], characterized by NMR spectroscopy, X-ray crystallography, and elemental analysis. Trichlorosilane reduction of [TBA]2[Si(P3O9)2] also provided anion 1. The electronic structures of 1 and 2 were investigated using a suite of theoretical methods; the computational studies suggest that the trichlorosilyl ligand is a good π-acceptor and forms σ-bonds with a high degree of s character.
中文翻译:

通过三氯甲硅烷基磷化物和硫化物阴离子形成 C-E (E = P, S) 键的正磷酸盐和硫酸盐的利用
用三氯硅烷还原磷酸 (H3PO4) 或四正丁基硫酸氢铵 ([TBA][HSO4]) 导致形成双(三氯甲硅烷基)磷化物 ([P(SiCl3)2]-, 1) 和三氯甲硅烷基硫化物 ( [Cl3SiS]-, 2) 阴离子。阴离子 1 和 2 的 TBA 盐形成的平衡方程是基于六氯二硅氧烷和氢气作为这些还原过程产生的副产物的鉴定而制定的:i) [H2PO4]- + 10HSiCl3 → 1 + 4O(SiCl3)2 + 6H2 用于 P 和 ii) [HSO4]- + 9HSiCl3 → 2 + 4O(SiCl3)2 + 5H2 用于 S。氢气是通过其随后使用 Crabtree's 氢化烯烃 ((-)-terpinen-4-ol) 而确定的催化剂 ([(COD)Ir(py)(PCy3)][PF6],COD = 1,5-环辛二烯,py = 吡啶,Cy = 环己基)。磷化物 1 是通过磷酸和三氯硅烷的反应原位生成的,用于将烷基氯(1-氯辛烷)转化为相应的伯膦,其以 41% 的产率分离。阴离子 1 也由 [TBA][H2PO4] 制备并以 62% 的克级产率分离。[TBA] 1 用过量的苄基氯处理导致形成四苄基氯化鏻,其以61%的产率分离。硫化物 2 用作硫化试剂,以 62% 的产率将二苯甲酮转化为噻二苯甲酮。它还以 55% 的产率将苄基溴转化为苄基硫醇。三偏磷酸 ([TBA]3[P3O9]·2H2O) 的 TBA 盐,也是阴离子 1 的前体,被发现与三氯硅烷或氯化硅 (IV) 反应生成双(三偏磷酸)硅酸盐 [TBA]2[ Si(P3O9)2], 通过核磁共振谱、X 射线晶体学和元素分析表征。[TBA]2[Si(P3O9)2] 的三氯硅烷还原也提供了阴离子 1。使用一套理论方法研究了 1 和 2 的电子结构;计算研究表明,三氯甲硅烷基配体是一种良好的 π 受体,并形成具有高度 s 特征的 σ 键。
更新日期:2019-03-22
中文翻译:

通过三氯甲硅烷基磷化物和硫化物阴离子形成 C-E (E = P, S) 键的正磷酸盐和硫酸盐的利用
用三氯硅烷还原磷酸 (H3PO4) 或四正丁基硫酸氢铵 ([TBA][HSO4]) 导致形成双(三氯甲硅烷基)磷化物 ([P(SiCl3)2]-, 1) 和三氯甲硅烷基硫化物 ( [Cl3SiS]-, 2) 阴离子。阴离子 1 和 2 的 TBA 盐形成的平衡方程是基于六氯二硅氧烷和氢气作为这些还原过程产生的副产物的鉴定而制定的:i) [H2PO4]- + 10HSiCl3 → 1 + 4O(SiCl3)2 + 6H2 用于 P 和 ii) [HSO4]- + 9HSiCl3 → 2 + 4O(SiCl3)2 + 5H2 用于 S。氢气是通过其随后使用 Crabtree's 氢化烯烃 ((-)-terpinen-4-ol) 而确定的催化剂 ([(COD)Ir(py)(PCy3)][PF6],COD = 1,5-环辛二烯,py = 吡啶,Cy = 环己基)。磷化物 1 是通过磷酸和三氯硅烷的反应原位生成的,用于将烷基氯(1-氯辛烷)转化为相应的伯膦,其以 41% 的产率分离。阴离子 1 也由 [TBA][H2PO4] 制备并以 62% 的克级产率分离。[TBA] 1 用过量的苄基氯处理导致形成四苄基氯化鏻,其以61%的产率分离。硫化物 2 用作硫化试剂,以 62% 的产率将二苯甲酮转化为噻二苯甲酮。它还以 55% 的产率将苄基溴转化为苄基硫醇。三偏磷酸 ([TBA]3[P3O9]·2H2O) 的 TBA 盐,也是阴离子 1 的前体,被发现与三氯硅烷或氯化硅 (IV) 反应生成双(三偏磷酸)硅酸盐 [TBA]2[ Si(P3O9)2], 通过核磁共振谱、X 射线晶体学和元素分析表征。[TBA]2[Si(P3O9)2] 的三氯硅烷还原也提供了阴离子 1。使用一套理论方法研究了 1 和 2 的电子结构;计算研究表明,三氯甲硅烷基配体是一种良好的 π 受体,并形成具有高度 s 特征的 σ 键。






























 京公网安备 11010802027423号
京公网安备 11010802027423号