当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Aerobic Oxidation of Cumene to Cumene Hydroperoxide over Mono- and Bimetallic Trimesate Metal–Organic Frameworks Prepared by a Facile “Green” Aqueous Synthesis
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-03-22 00:00:00 , DOI: 10.1021/acssuschemeng.8b06472 Anna Nowacka 1 , Pol Briantais 2 , Carmelo Prestipino 2 , Francesc X. Llabrés i Xamena 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-03-22 00:00:00 , DOI: 10.1021/acssuschemeng.8b06472 Anna Nowacka 1 , Pol Briantais 2 , Carmelo Prestipino 2 , Francesc X. Llabrés i Xamena 1
Affiliation
|
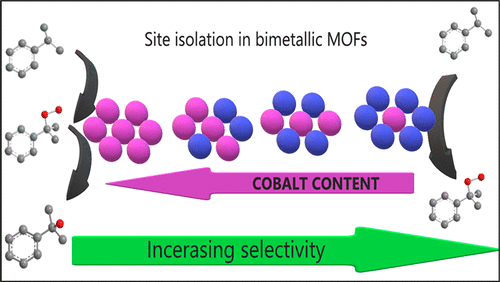
Co–Ni and Mn–Ni bimetallic trimesate MOFs prepared by a fast aqueous synthesis method are excellent and reusable catalysts for the selective aerobic oxidation of cumene to cumene hydroperoxide (CHP). Isolation of Co2+ (or Mn2+) in an inert Ni-BTC framework is a good strategy to optimize CHP selectivity above 90%: since only Co2+ sites catalyze CHP decomposition, a drop of the CHP selectivity is observed as the cobalt content in the bimetallic MOF increases. The statistical probability of having isolated Co2+ sites is calculated as a function of the total cobalt content of the bimetallic compound, assuming homogeneous distribution of Co2+ ions in the Ni-BTC framework and preferential occupation of terminal sites. Thus, in our best sample, with a Co:Ni ratio of 5:95, 73% of the total Co2+ ions are isolated so that CHP decomposition/overoxidation processes at the surface of the catalyst are not likely to occur before CHP desorption. This can explain the excellent CHP selectivity (91%) attained over this material. This “site isolation” effect is further supported by similar findings on Mn–Ni bimetallic compounds.
中文翻译:

通过简便的“绿色”水合成制备的单金属和双金属三酸酯金属-有机骨架上的异丙苯选择性好氧氧化为氢过氧化异丙苯
通过快速水合成方法制备的Co-Ni和Mn-Ni双金属偏苯三酸酯MOF是出色的可重复使用的催化剂,可用于将异丙苯好氧氧化为氢过氧化枯烯(CHP)。在惰性Ni-BTC框架中分离Co 2+(或Mn 2+)是优化CHP选择性高于90%的好策略:由于仅Co 2+位点催化CHP分解,因此随着CHP选择性的降低,CHP选择性下降。双金属MOF中的钴含量增加。假设Co 2+分布均匀,则计算出具有孤立Co 2+位点的统计概率是双金属化合物总钴含量的函数Ni-BTC框架中的离子和优先占用终端站点。因此,在我们最好的样品中,Co:Ni比率为5:95,可分离出总Co 2+离子的73%,因此在CHP脱附之前不太可能在催化剂表面发生CHP分解/过氧化过程。 。这可以解释在该材料上获得的出色的CHP选择性(91%)。锰镍双金属化合物的类似发现进一步支持了这种“位隔离”效应。
更新日期:2019-03-22
中文翻译:

通过简便的“绿色”水合成制备的单金属和双金属三酸酯金属-有机骨架上的异丙苯选择性好氧氧化为氢过氧化异丙苯
通过快速水合成方法制备的Co-Ni和Mn-Ni双金属偏苯三酸酯MOF是出色的可重复使用的催化剂,可用于将异丙苯好氧氧化为氢过氧化枯烯(CHP)。在惰性Ni-BTC框架中分离Co 2+(或Mn 2+)是优化CHP选择性高于90%的好策略:由于仅Co 2+位点催化CHP分解,因此随着CHP选择性的降低,CHP选择性下降。双金属MOF中的钴含量增加。假设Co 2+分布均匀,则计算出具有孤立Co 2+位点的统计概率是双金属化合物总钴含量的函数Ni-BTC框架中的离子和优先占用终端站点。因此,在我们最好的样品中,Co:Ni比率为5:95,可分离出总Co 2+离子的73%,因此在CHP脱附之前不太可能在催化剂表面发生CHP分解/过氧化过程。 。这可以解释在该材料上获得的出色的CHP选择性(91%)。锰镍双金属化合物的类似发现进一步支持了这种“位隔离”效应。













































 京公网安备 11010802027423号
京公网安备 11010802027423号