当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling Substrate Binding to Fe4S4 Clusters through Remote Steric Effects
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-03-22 00:00:00 , DOI: 10.1021/acs.inorgchem.9b00360
Alexandra C. Brown 1 , Daniel L. M. Suess 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-03-22 00:00:00 , DOI: 10.1021/acs.inorgchem.9b00360
Alexandra C. Brown 1 , Daniel L. M. Suess 1
Affiliation
|
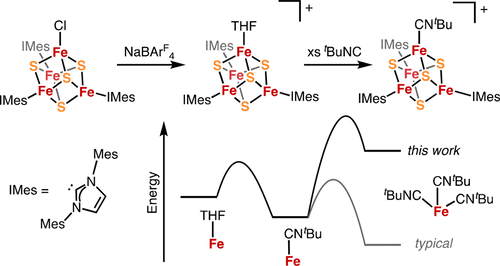
The extraordinary reactivity exhibited by many Fe–S enzymes is due in large part to the influence of the protein scaffold on substrate binding and activation. In principle, the coordination chemistry of synthetic Fe–S clusters could similarly be controlled through remote steric effects. Toward this end, we report the synthesis of 3:1 site-differentiated [Fe4S4] clusters ligated by N-heterocyclic carbene (NHC) ligands with variable steric profiles: IMes (1,3-dimesitylimidazol-2-ylidene) and IiPrMe (1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene). Treatment of (IMes)3Fe4S4Cl with NaBArF4 in ethereal solvents (Et2O and THF) leads to the formation of an ether adduct, [(IMes)3Fe4S4(solv)][BArF4]; solvent can be displaced by addition of tBuNC to form the unusual monoisocyanide adduct [(IMes)3Fe4S4(CNtBu)][BArF4]. Carrying out the same reactions with the less sterically encumbered cluster (IiPrMe)3Fe4S4Cl results in more typical reactivity: undesired ligand redistribution to form the homoleptic cluster [(IiPrMe)4Fe4S4][BArF4] and generation of the triisocyanide adduct [(IiPrMe)3Fe4S4(CNtBu)3][BArF4]. The increased steric profile of the IMes ligands disfavors ligand redistribution and defines a binding pocket at the apical Fe, thereby enabling the generation of a coordinatively unsaturated and substitutionally labile Fe site. This method of controlling the coordination chemistry at the apical Fe site by modifying the sterics of ligands bound to adjacent Fe sites complements existing strategies for generating site-differentiated Fe–S clusters and provides new opportunities to direct reactivity at cuboidal metalloclusters.
中文翻译:

通过远程立体效应控制底物与Fe 4 S 4团簇的结合
许多Fe–S酶表现出的非凡反应性在很大程度上是由于蛋白质支架对底物结合和激活的影响。原则上,合成Fe–S团簇的配位化学可以类似地通过远程空间效应来控制。为此目的,我们报告的合成3:1站点分化的[Fe 4小号4 ]用N连接的簇-杂环卡宾(NHC)配体具有可变的位配置文件:IMES(1,3- dimesitylimidazol -2-亚基)和我我镨我(1,3-二异丙基-4,5-二甲基咪唑烷-2-亚基)。NaBAr F 4处理(IMes)3 Fe 4 S 4 Cl在醚溶剂(Et 2 O和THF)中形成醚加合物,[(IMes)3 Fe 4 S 4(solv)] [BAr F 4 ];可以通过添加t BuNC来置换溶剂,以形成不寻常的单异氰酸酯加合物[(IMes)3 Fe 4 S 4(CN t Bu)] [BAr F 4 ]。对空间较小的簇(I i Pr Me)3 Fe 4 S 4进行相同的反应Cl导致更典型的反应性:不希望的配体重新分布以形成均聚簇[[I i Pr Me)4 Fe 4 S 4 ] [BAr F 4 ],并生成三异氰酸酯加合物[(I i Pr Me)3 Fe 4 S 4(CN t Bu)3 ] [BAr F 4]。IMes配体的增加的空间分布不利于配体的重新分布,并在顶端的Fe处限定了一个结合口袋,从而能够产生配位不饱和且不稳定的Fe位点。这种通过改变与相邻Fe位点结合的配位体的空间来控制顶部Fe位点上的配位化学的方法,可补充现有的生成位点分化的Fe–S团簇的策略,并为在立方体金属簇上直接反应提供了新的机会。
更新日期:2019-03-22
中文翻译:

通过远程立体效应控制底物与Fe 4 S 4团簇的结合
许多Fe–S酶表现出的非凡反应性在很大程度上是由于蛋白质支架对底物结合和激活的影响。原则上,合成Fe–S团簇的配位化学可以类似地通过远程空间效应来控制。为此目的,我们报告的合成3:1站点分化的[Fe 4小号4 ]用N连接的簇-杂环卡宾(NHC)配体具有可变的位配置文件:IMES(1,3- dimesitylimidazol -2-亚基)和我我镨我(1,3-二异丙基-4,5-二甲基咪唑烷-2-亚基)。NaBAr F 4处理(IMes)3 Fe 4 S 4 Cl在醚溶剂(Et 2 O和THF)中形成醚加合物,[(IMes)3 Fe 4 S 4(solv)] [BAr F 4 ];可以通过添加t BuNC来置换溶剂,以形成不寻常的单异氰酸酯加合物[(IMes)3 Fe 4 S 4(CN t Bu)] [BAr F 4 ]。对空间较小的簇(I i Pr Me)3 Fe 4 S 4进行相同的反应Cl导致更典型的反应性:不希望的配体重新分布以形成均聚簇[[I i Pr Me)4 Fe 4 S 4 ] [BAr F 4 ],并生成三异氰酸酯加合物[(I i Pr Me)3 Fe 4 S 4(CN t Bu)3 ] [BAr F 4]。IMes配体的增加的空间分布不利于配体的重新分布,并在顶端的Fe处限定了一个结合口袋,从而能够产生配位不饱和且不稳定的Fe位点。这种通过改变与相邻Fe位点结合的配位体的空间来控制顶部Fe位点上的配位化学的方法,可补充现有的生成位点分化的Fe–S团簇的策略,并为在立方体金属簇上直接反应提供了新的机会。

































 京公网安备 11010802027423号
京公网安备 11010802027423号