当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Can Ni Complexes Behave as Molecular Water Oxidation Catalysts?
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-03-24 00:00:00 , DOI: 10.1021/acscatal.8b03953 Pablo Garrido-Barros 1, 2 , Sergi Grau 1 , Samuel Drouet 1 , Jordi Benet-Buchholz 1 , Carolina Gimbert-Suriñach 1 , Antoni Llobet 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-03-24 00:00:00 , DOI: 10.1021/acscatal.8b03953 Pablo Garrido-Barros 1, 2 , Sergi Grau 1 , Samuel Drouet 1 , Jordi Benet-Buchholz 1 , Carolina Gimbert-Suriñach 1 , Antoni Llobet 1, 3
Affiliation
|
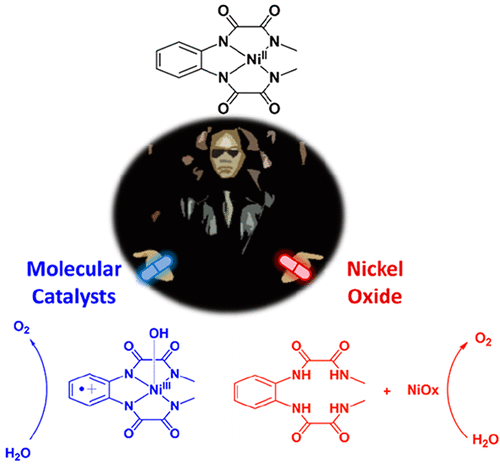
The present report uncovers the borderline between homogeneous and heterogeneous water oxidation catalysis using a family of Ni complexes containing oxamidate anionic type of ligands. In particular, the Ni complex [(L1)NiII]2– (12–; L1 = o-phenylenebis(oxamidate)) and its modified analogues [(L2)NiII]2– (22–; L2 = 4,5-dimethyl-1,2-phenylenebis(oxamidate)) and [(L3)NiII]2– (32–; L3 = 4-methoxy-1,2-phenylenebis(oxamidate)) have been prepared and evaluated as molecular water oxidation catalysts at basic pH. Their redox features have been analyzed by means of electrochemical measurements revealing a crucial involvement of the ligand in the electron transfer processes. Moreover, the stability of those complexes has been assessed both in solution and immobilized on graphene-based electrodes at different potentials and pHs. The degradation of the molecular species generates a NiOx (Niquel oxides of general formula NixOyHz) layer, whose stability and activity as water oxidation catalyst have also been established. Electrochemical methods, together with surface characterization techniques, have shown the complex mechanistic scenario in water oxidation catalyzed by this family of Ni complexes, which consists of the coexistence of two catalytic mechanisms: a homogeneous pathway driven by the molecular complex and a heterogeneous pathway based on NiOx. The electronic perturbations exerted through the ligand framework have manifested a strong influence over the stability of the molecular species under turnover conditions. Finally, 12– has been used as a molecular precursor for the formation of NiFeOx (Niquel/Iron oxides of general formula NixFe1-xOyHz) anodes that behave as extremely powerful water oxidation anodes.
中文翻译:

镍配合物可以用作分子水氧化催化剂吗?
本报告揭示了使用含草酰胺酸阴离子型配体的镍配合物族在均相和非均相水氧化催化之间的边界。特别是Ni络合物[(L 1)Ni II ] 2–(1 2– ; L 1 =邻苯二甲双(草酰胺))及其修饰的类似物[(L 2)Ni II ] 2–(2 2– ; L 2 = 4,5-二甲基-1,2-亚苯基双(草酰胺酸酯))和[(L 3)Ni II ] 2–(3 2– ; L 3制备了4-甲氧基-1,2-亚苯基双(草酰胺酸酯),并在碱性pH下作为分子水氧化催化剂进行了评估。已经通过电化学测量对它们的氧化还原特征进行了分析,揭示了配体在电子转移过程中的关键参与。此外,已经在溶液中以及在不同电势和pH值下固定在石墨烯基电极上评估了这些络合物的稳定性。分子种类的降解产生NiOx(通式Ni x O y H z的一氧化氮层,其稳定性和作为水氧化催化剂的活性也已建立。电化学方法以及表面表征技术已经表明,该镍配合物家族催化水氧化的机理很复杂,包括两种催化机制的共存:分子配合物驱动的均相途径和基于氢氧根的异质途径。氧化镍 通过配体骨架施加的电子扰动已显示出在翻转条件下对分子种类的稳定性的强烈影响。最后,1 2-被用作形成NiFeOx的分子前体(通式为Ni x Fe 1-x O y的Niquel /氧化铁H z)阳极,具有极强的水氧化阳极性能。
更新日期:2019-03-24
中文翻译:

镍配合物可以用作分子水氧化催化剂吗?
本报告揭示了使用含草酰胺酸阴离子型配体的镍配合物族在均相和非均相水氧化催化之间的边界。特别是Ni络合物[(L 1)Ni II ] 2–(1 2– ; L 1 =邻苯二甲双(草酰胺))及其修饰的类似物[(L 2)Ni II ] 2–(2 2– ; L 2 = 4,5-二甲基-1,2-亚苯基双(草酰胺酸酯))和[(L 3)Ni II ] 2–(3 2– ; L 3制备了4-甲氧基-1,2-亚苯基双(草酰胺酸酯),并在碱性pH下作为分子水氧化催化剂进行了评估。已经通过电化学测量对它们的氧化还原特征进行了分析,揭示了配体在电子转移过程中的关键参与。此外,已经在溶液中以及在不同电势和pH值下固定在石墨烯基电极上评估了这些络合物的稳定性。分子种类的降解产生NiOx(通式Ni x O y H z的一氧化氮层,其稳定性和作为水氧化催化剂的活性也已建立。电化学方法以及表面表征技术已经表明,该镍配合物家族催化水氧化的机理很复杂,包括两种催化机制的共存:分子配合物驱动的均相途径和基于氢氧根的异质途径。氧化镍 通过配体骨架施加的电子扰动已显示出在翻转条件下对分子种类的稳定性的强烈影响。最后,1 2-被用作形成NiFeOx的分子前体(通式为Ni x Fe 1-x O y的Niquel /氧化铁H z)阳极,具有极强的水氧化阳极性能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号