Nature Reviews Chemistry ( IF 38.1 ) Pub Date : 2019-03-25 , DOI: 10.1038/s41570-019-0087-1 Ron Naaman , Yossi Paltiel , David H. Waldeck
|
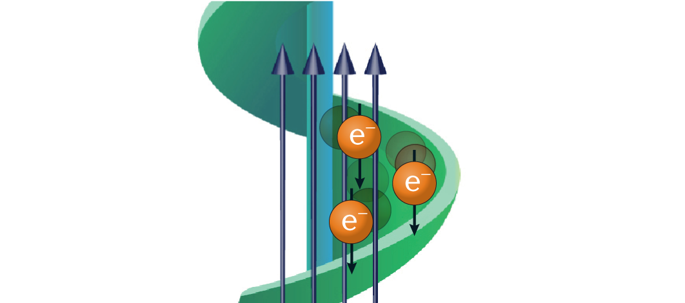
The electron’s spin is essential to the stability of matter, and control over the spin opens up avenues for manipulating the properties of molecules and materials. The Pauli exclusion principle requires that two electrons in a single spatial eigenstate have opposite spins, and this fact dictates basic features of atomic states and chemical bond formation. The energy associated with interacting electron clouds changes with their relative spin orientation, and by manipulating the spin directions, one can guide chemical transformations. However, controlling the relative spin orientation of electrons located on two reactants (atoms, molecules or surfaces) has proved challenging. Recent developments based on the chiral-induced spin selectivity (CISS) effect show that the spin orientation is linked to molecular symmetry and can be controlled in ways not previously imagined. For example, the combination of chiral molecules and electron spin opens up a new approach to (enantio)selective chemistry. This Review describes the theoretical concepts underlying the CISS effect and illustrates its importance by discussing some of its manifestations in chemistry, biology and physics. Specifically, we discuss how the CISS effect allows for efficient long-range electron transfer in chiral molecules and how it affects biorecognition processes. Several applications of the effect are presented, and the importance of controlling relative spin orientations in multi-electron processes, such as electrochemical water splitting, is emphasized. We describe the enantiospecific interaction between ferromagnetic substrates and chiral molecules and how it enables the separation of enantiomers with ferromagnets. Lastly, we discuss the relevance of CISS effects to biological electron transfer, enantioselectivity and CISS-based spintronics applications.
中文翻译:

手性分子与电子自旋
电子的自旋对于物质的稳定性至关重要,对电子自旋的控制为操纵分子和材料的性质开辟了道路。保利排斥原理要求单个空间本征态中的两个电子具有相反的自旋,这一事实决定了原子态和化学键形成的基本特征。与相互作用的电子云相关的能量随其相对自旋方向而变化,并且通过操纵自旋方向,可以指导化学转化。然而,事实证明,控制位于两种反应物(原子,分子或表面)上的电子的相对自旋取向具有挑战性。基于手性诱导的自旋选择性(CISS)效应的最新进展表明,自旋取向与分子对称性相关,可以通过以前未曾想象的方式进行控制。例如,手性分子和电子自旋的结合开辟了一种新的(对映)选择性化学方法。这篇综述描述了CISS效应的基本理论概念,并通过讨论化学,生物学和物理学中的一些表现来说明其重要性。具体来说,我们讨论了CISS效应如何在手性分子中实现有效的远程电子转移,以及它如何影响生物识别过程。介绍了该效应的几种应用,并强调了在多电子过程(例如电化学水分解)中控制相对自旋取向的重要性。我们描述了铁磁底物和手性分子之间的对映体特异性相互作用,以及它如何使对映体与铁磁体分离。最后,我们讨论了CISS效应与生物电子转移,对映选择性和基于CISS的自旋电子学应用的相关性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号