当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combining Chiral Aldehyde Catalysis and Transition-Metal Catalysis for Enantioselective α-Allylic Alkylation of Amino Acid Esters
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-03-21 , DOI: 10.1021/jacs.9b01910 Lei Chen 1 , Ming-Jing Luo 1 , Fang Zhu 1 , Wei Wen 1 , Qi-Xiang Guo 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-03-21 , DOI: 10.1021/jacs.9b01910 Lei Chen 1 , Ming-Jing Luo 1 , Fang Zhu 1 , Wei Wen 1 , Qi-Xiang Guo 1
Affiliation
|
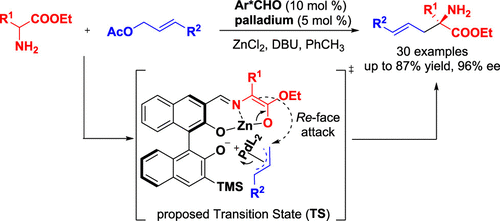
A chiral aldehyde is rationally combined with a Lewis acid and a transition metal for the first time to form a triple catalytic system. This cocatalytic system exhibits good catalytic activation and stereoselective-control abilities in the asymmetric α-allylation reaction of N-unprotected amino acid esters and allyl acetates. Optically active α,α-disubstituted α-amino acids (α-AAs) are generated in good yields (up to 87%) and enantioselectivities (up to 96% ee). Preliminary mechanism investigation indicates that the chiral aldehyde 3f acts both as an organocatalyst to activate the amino acid ester via the formation of a Schiff base, and as a ligand to facilitate the nucleophilic attack process by coordinating with π-allyl Pd(II) species.
中文翻译:

结合手性醛催化和过渡金属催化对氨基酸酯的对映选择性α-烯丙基烷基化
手性醛首次与路易斯酸和过渡金属合理结合,形成三重催化体系。该助催化体系在 N-未保护氨基酸酯和乙酸烯丙酯的不对称 α-烯丙基化反应中表现出良好的催化活化和立体选择性控制能力。以良好的产率(高达 87%)和对映选择性(高达 96% ee)生成具有光学活性的 α,α-二取代 α-氨基酸 (α-AAs)。初步机制研究表明,手性醛 3f 既可作为有机催化剂通过形成席夫碱来激活氨基酸酯,也可作为配体通过与 π-烯丙基 Pd(II) 物种配位促进亲核攻击过程。
更新日期:2019-03-21
中文翻译:

结合手性醛催化和过渡金属催化对氨基酸酯的对映选择性α-烯丙基烷基化
手性醛首次与路易斯酸和过渡金属合理结合,形成三重催化体系。该助催化体系在 N-未保护氨基酸酯和乙酸烯丙酯的不对称 α-烯丙基化反应中表现出良好的催化活化和立体选择性控制能力。以良好的产率(高达 87%)和对映选择性(高达 96% ee)生成具有光学活性的 α,α-二取代 α-氨基酸 (α-AAs)。初步机制研究表明,手性醛 3f 既可作为有机催化剂通过形成席夫碱来激活氨基酸酯,也可作为配体通过与 π-烯丙基 Pd(II) 物种配位促进亲核攻击过程。






























 京公网安备 11010802027423号
京公网安备 11010802027423号