当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
tert‐Butoxide‐Mediated Synthesis of 3,4′‐Biquinolines from 2‐Aminochalcones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-04-10 , DOI: 10.1002/adsc.201900029
Jiye Jeon 1 , So Young Lee 1 , Cheol‐Hong Cheon 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-04-10 , DOI: 10.1002/adsc.201900029
Jiye Jeon 1 , So Young Lee 1 , Cheol‐Hong Cheon 1
Affiliation
|
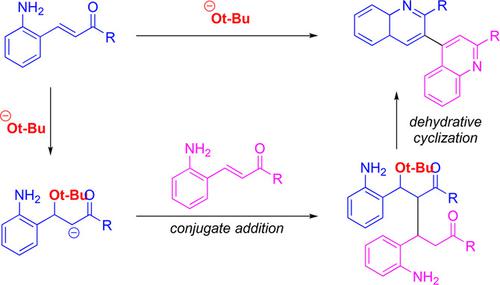
A novel protocol to synthesize 3,4’‐biquinolines from 2‐aminochalcones in the presence of a stoichiometric amount of sodium tert‐butoxide as the nucleophilic promotor was developed. Conjugate addition of tert‐butoxide to 2‐aminochalcones provided the corresponding enolates, which underwent Michael addition to another molecule of 2‐aminochalcone to afford a dimeric species of 2‐aminochalcones. Subsequent cyclization between the amino and carbonyl groups followed by aromatization provides 3,4’‐biquinoline products. Various 2‐aminochalcones were submitted to this protocol and the desired 3,4’‐biquinoline products were obtained in good to high yields in a short reaction time.
中文翻译:

叔丁氧化物介导的2-氨基min酮合成3,4'-联喹啉
开发了一种新颖的方案,可以在化学计量的叔丁醇钠作为亲核促进剂的情况下,从2-氨基查耳酮合成3,4'-喹啉。将叔丁醇盐与2-氨基查耳酮共轭可提供相应的烯醇盐,然后将迈克尔加成至另一个2-氨基查耳酮分子中,即可得到2-氨基查耳酮的二聚体。随后在氨基和羰基之间进行环化,然后进行芳构化,得到3,4'-联喹啉产物。各种2-氨基查耳酮均已提交至该方案,并在短时间内以良好或高收率获得了所需的3,4'-联喹啉产品。
更新日期:2019-04-10
中文翻译:

叔丁氧化物介导的2-氨基min酮合成3,4'-联喹啉
开发了一种新颖的方案,可以在化学计量的叔丁醇钠作为亲核促进剂的情况下,从2-氨基查耳酮合成3,4'-喹啉。将叔丁醇盐与2-氨基查耳酮共轭可提供相应的烯醇盐,然后将迈克尔加成至另一个2-氨基查耳酮分子中,即可得到2-氨基查耳酮的二聚体。随后在氨基和羰基之间进行环化,然后进行芳构化,得到3,4'-联喹啉产物。各种2-氨基查耳酮均已提交至该方案,并在短时间内以良好或高收率获得了所需的3,4'-联喹啉产品。

































 京公网安备 11010802027423号
京公网安备 11010802027423号