European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-03-21 , DOI: 10.1016/j.ejmech.2019.03.044 Ying Chen , Bolin Wu , Yameng Hao , Yunqi Liu , Zhili Zhang , Chao Tian , Xianling Ning , Ying Guo , Junyi Liu , Xiaowei Wang
|
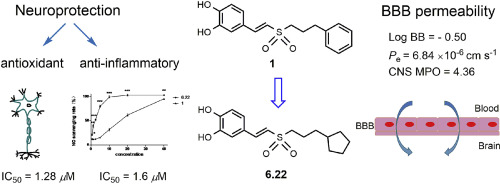
(E)-3,4-dihydroxystyryl alkyl sulfones, as new analogues of neurodegenerative agents, were designed and synthesized. The biological results demonstrated that most of the target compounds preserved antioxidant and anti-inflammatory potency in scavenging reactive free radicals, protecting neuronal cells against neurotoxins such as H2O2, 6-hydroxydopamine and inhibiting lipopolysaccharide (LPS)-induced over-production of NO. Among these compounds, 6.22 with cyclopentyl propyl exhibited prominent antioxidant activity at low concentration (2.5 μM) in H2O2 model (cell viability = 94.5%). In addition, 6.22 (IC50 = 1.6 μM) displayed better anti-inflammatory activity than that of lead compound 1 (IC50 = 13.4 μM). In view of the outstanding performance of 6.22, the apoptotic rates of H2O2-damaged PC12 cells were detected by Annexin V-FITC/PI assay. 6.22 showed higher potency in inhibition of apoptosis than 1 at low concentration (2.5 μM), consisting with the antioxidant and anti-inflammatory models. Furthermore, with the predicted CNS (+) blood-brain barrier (BBB) permeability (Pe = 6.84 × 10−6 cm s−1), low cytotoxicity and favorable physiochemical properties based on calculation, compound 6.22 can be further developed as a potential multifunctional neuroprotective agent.
中文翻译:

(E)-3,4-二羟基苯乙烯基烷基砜作为新型神经保护剂的结构-活性关系研究基于改进的抗氧化剂,抗炎活性和血脑屏障通透性
设计并合成了(E)-3,4-二羟基苯乙烯基烷基砜,作为神经变性剂的新类似物。生物结果表明,大部分目标化合物在清除活性自由基保留的抗氧化和抗炎效力,保护神经元细胞免于神经毒素如H 2 ö 2,6-羟基和抑制脂多糖(LPS)诱导的过度产生的不。在这些化合物中,6.22在低浓度(2.5环戊基丙基展出突出的抗氧化活性 μ H中M)2 ö 2模型(细胞活力= 94.5%)。此外,6.22(IC 50 = 1.6 μ M)显示比铅化合物的更好的抗炎活性1(IC 50 = 13.4 μ M)。鉴于6.22的优异性能,通过膜联蛋白V-FITC / PI测定法检测了H 2 O 2损伤的PC12细胞的凋亡率。6.22显示出更高效力在抑制细胞凋亡比1 在低浓度(2.5 μ M),其由与抗氧化剂和抗炎模型。此外,在具有预测的CNS(+)血脑屏障(BBB)渗透性的情况下(P e = 6.84×10 -6 cm s -1),低细胞毒性和良好的理化性质(基于计算),化合物6.22可以进一步开发为潜在的多功能神经保护剂。

































 京公网安备 11010802027423号
京公网安备 11010802027423号