Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational Analysis of the Supramolecular Complexation of Diaryl-Substituted Tetrathiafulvalene Vinylogues with Fullerenes
ACS Omega ( IF 3.7 ) Pub Date : 2019-03-21 00:00:00 , DOI: 10.1021/acsomega.9b00065 Ahmad I Alrawashdeh 1 , Yuming Zhao 1 , Jolanta B Lagowski 1
ACS Omega ( IF 3.7 ) Pub Date : 2019-03-21 00:00:00 , DOI: 10.1021/acsomega.9b00065 Ahmad I Alrawashdeh 1 , Yuming Zhao 1 , Jolanta B Lagowski 1
Affiliation
|
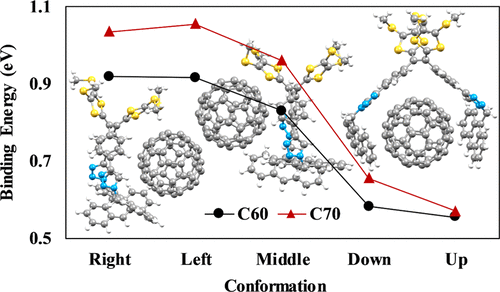
Tetrathiafulvalene vinylogues (TTFVs) functionalized with diaryl substituents (aryl = 1-napthyl, 9-anthryl, and 1-pyrenyl) via click chemistry have been previously synthesized and studied as tweezer-type receptors for binding with C60 and C70 fullerenes. In particular, dianthryl-TTFV exhibits unique selectivity for C70 fullerene, giving rise to effective fluorescence turn-on sensing of C70 in the presence of a large excess of C60 fullerene. This observation indicated that dianthryl-TTFV has a preferential binding affinity for C70 over C60 fullerene, but the reason for such selectivity is unclear. Aiming at addressing this issue, we herein investigated the relative conformational stability of diaryl-substituted TTFVs in complexation with C70 and C60 fullerenes. The dispersion-corrected density functional theory approximation (B3LYP-D3) was employed in our computational analysis to determine binding energies and electronic properties of these supramolecular complexes. It was found that the highest binding energies (and the lowest relative conformational energies) are in pairings when fullerenes are placed around the central TTFV moieties (such as the triazole rings). The results of electronic properties show that the dianthryl-TTFV and dipyrenyl-TTFV conformers have lower highest occupied molecular orbital–lowest unoccupied molecular orbital gaps relative to the ones obtained for dinaphthyl-TTFV, indicating that dianthryl-TTFV, and to some extend dipyrenyl-TTFV, could be good candidates for chemical sensing of fullerenes with fluorescence spectroscopy. We also investigated the effect of the solvent on the interactions of the diaryl-TTFVs with fullerenes using the polarizable continuum model. In general, the presence of a solvent decreases the diaryl-TTFV/fullerene binding energies, presumably because of the interactions of the solvent with individual fullerenes and diaryl-TTFVs.
中文翻译:

二芳基取代的四硫富瓦烯乙烯系物与富勒烯的超分子络合的构象分析
之前已经合成并研究了通过点击化学用二芳基取代基(芳基 = 1-萘基、9-蒽基和 1-芘基)功能化的四硫富瓦烯乙烯系物 (TTFV),作为与 C 60 和 C 70 富勒烯结合的镊子型受体。特别地,二蒽基-TTFV对C 70富勒烯表现出独特的选择性,在存在大量过量的C 60富勒烯的情况下产生对C 70的有效荧光开启感测。该观察表明,与C 60富勒烯相比,二蒽基-TTFV 对C 70具有优先的结合亲和力,但这种选择性的原因尚不清楚。为了解决这个问题,我们在此研究了二芳基取代的TTFV与C 70和C 60富勒烯络合的相对构象稳定性。我们的计算分析中采用色散校正密度泛函理论近似(B3LYP-D3)来确定这些超分子复合物的结合能和电子特性。研究发现,当富勒烯位于中心 TTFV 部分(例如三唑环)周围时,最高结合能(和最低相对构象能)成对出现。电子性质结果表明,与二萘基-TTFV相比,二蒽基-TTFV和二芘基-TTFV构象异构体具有较低的最高占据分子轨道-最低未占据分子轨道间隙,这表明二蒽基-TTFV和在某种程度上二芘基- TTFV 可能是利用荧光光谱对富勒烯进行化学传感的良好候选者。我们还使用可极化连续介质模型研究了溶剂对二芳基-TTFV 与富勒烯相互作用的影响。一般来说,溶剂的存在会降低二芳基-TTFV/富勒烯的结合能,可能是因为溶剂与单个富勒烯和二芳基-TTFV的相互作用。
更新日期:2019-03-21
中文翻译:

二芳基取代的四硫富瓦烯乙烯系物与富勒烯的超分子络合的构象分析
之前已经合成并研究了通过点击化学用二芳基取代基(芳基 = 1-萘基、9-蒽基和 1-芘基)功能化的四硫富瓦烯乙烯系物 (TTFV),作为与 C 60 和 C 70 富勒烯结合的镊子型受体。特别地,二蒽基-TTFV对C 70富勒烯表现出独特的选择性,在存在大量过量的C 60富勒烯的情况下产生对C 70的有效荧光开启感测。该观察表明,与C 60富勒烯相比,二蒽基-TTFV 对C 70具有优先的结合亲和力,但这种选择性的原因尚不清楚。为了解决这个问题,我们在此研究了二芳基取代的TTFV与C 70和C 60富勒烯络合的相对构象稳定性。我们的计算分析中采用色散校正密度泛函理论近似(B3LYP-D3)来确定这些超分子复合物的结合能和电子特性。研究发现,当富勒烯位于中心 TTFV 部分(例如三唑环)周围时,最高结合能(和最低相对构象能)成对出现。电子性质结果表明,与二萘基-TTFV相比,二蒽基-TTFV和二芘基-TTFV构象异构体具有较低的最高占据分子轨道-最低未占据分子轨道间隙,这表明二蒽基-TTFV和在某种程度上二芘基- TTFV 可能是利用荧光光谱对富勒烯进行化学传感的良好候选者。我们还使用可极化连续介质模型研究了溶剂对二芳基-TTFV 与富勒烯相互作用的影响。一般来说,溶剂的存在会降低二芳基-TTFV/富勒烯的结合能,可能是因为溶剂与单个富勒烯和二芳基-TTFV的相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号