当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elevated reaction order of 1,3,5-tri-tert-butylbenzene bromination as evidence of a clustered polybromide transition state: a combined kinetic and computational study
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2019-03-20 , DOI: 10.1039/c9ob00607a Andrey V. Shernyukov 1, 2, 3, 3, 4 , Alexander M. Genaev 1, 2, 3 , George E. Salnikov 1, 2, 3, 3, 4 , Vyacheslav G. Shubin 1, 2, 3 , Henry S. Rzepa 5, 6, 7, 8
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2019-03-20 , DOI: 10.1039/c9ob00607a Andrey V. Shernyukov 1, 2, 3, 3, 4 , Alexander M. Genaev 1, 2, 3 , George E. Salnikov 1, 2, 3, 3, 4 , Vyacheslav G. Shubin 1, 2, 3 , Henry S. Rzepa 5, 6, 7, 8
Affiliation
|
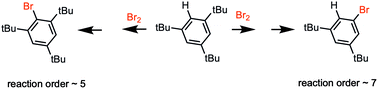
The kinetics and mechanism of concurrent bromo-de-protonation and bromo-de-tert-butylation of 1,3,5-tri-tert-butylbenzene at different bromine concentrations were studied experimentally and theoretically. Both reactions have high order in bromine (experimental kinetic orders ∼5 and ∼7, respectively). According to quantum chemical DFT calculations, such high reaction orders are caused by participation of clustered polybromide anions Br2n−1– in transition states. Bromo-de-tert-butylation has a higher order due to its bigger reaction center demanding clusters of extended size. A significant primary deuterium kinetic isotope effect (KIE) for bromo-de-protonation is measured indicating proton removal is rate limiting, as confirmed by computed DFT models. The latter predict a larger value for the KIE than measured and possible explanations for this are discussed.
中文翻译:

1,3,5-三叔丁基苯溴化反应的顺序升高,证明了聚溴化物过渡态的聚集:动力学和计算研究的组合
通过理论和实验研究了1,3,5-三叔丁基苯在不同溴浓度下同时进行溴脱质子化和溴脱叔丁基化的动力学和机理。两种反应在溴中都具有较高的阶数(实验动力学阶数分别为〜5和〜7)。根据量子化学DFT计算,如此高的反应阶数是由于簇状多溴化物阴离子Br 2 n -1 –参与过渡态引起的。溴去叔丁基化反应由于其较大的反应中心而需要更高尺寸的簇,因此具有更高的阶数。如通过计算的DFT模型所证实的,对溴去质子化的显着初级氘动力学同位素效应(KIE)进行了测量,表明质子的去除是速率限制。后者预测的KIE值比测量的值大,并讨论了对此的可能解释。
更新日期:2019-04-11
中文翻译:

1,3,5-三叔丁基苯溴化反应的顺序升高,证明了聚溴化物过渡态的聚集:动力学和计算研究的组合
通过理论和实验研究了1,3,5-三叔丁基苯在不同溴浓度下同时进行溴脱质子化和溴脱叔丁基化的动力学和机理。两种反应在溴中都具有较高的阶数(实验动力学阶数分别为〜5和〜7)。根据量子化学DFT计算,如此高的反应阶数是由于簇状多溴化物阴离子Br 2 n -1 –参与过渡态引起的。溴去叔丁基化反应由于其较大的反应中心而需要更高尺寸的簇,因此具有更高的阶数。如通过计算的DFT模型所证实的,对溴去质子化的显着初级氘动力学同位素效应(KIE)进行了测量,表明质子的去除是速率限制。后者预测的KIE值比测量的值大,并讨论了对此的可能解释。






























 京公网安备 11010802027423号
京公网安备 11010802027423号