当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective cleavage of lignin and lignin model compounds without external hydrogen, catalyzed by heterogeneous nickel catalysts†
Chemical Science ( IF 7.6 ) Pub Date : 2019-03-21 00:00:00 , DOI: 10.1039/c9sc00691e
Liang Jiang 1 , Haiwei Guo 2, 3 , Changzhi Li 2 , Peng Zhou 1 , Zehui Zhang 1
Chemical Science ( IF 7.6 ) Pub Date : 2019-03-21 00:00:00 , DOI: 10.1039/c9sc00691e
Liang Jiang 1 , Haiwei Guo 2, 3 , Changzhi Li 2 , Peng Zhou 1 , Zehui Zhang 1
Affiliation
|
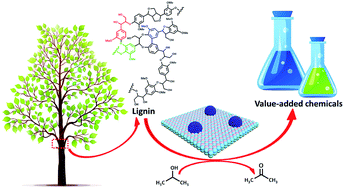
Selective hydrogenolysis of the Caryl–O bonds in lignin is a key strategy for the generation of fuels and chemical feedstocks from biomass. Currently, hydrogenolysis has been mainly conducted using hydrogen, which is flammable and not sustainable or economical. Herein, an external hydrogen-free process for aryl ethers hydrogenolysis in lignin models and dioxasolv lignin over nickel nanoparticles supported on Al2O3, is reported. Kinetic studies reveal that the transfer hydrogenolysis activity of the three model compounds decreased in the following order: benzyl phenyl ether (α-O-4), 2-phenylethyl phenyl ether (β-O-4) and diphenyl ether (4-O-5), which linearly corresponds to their binding energies and the activation energies. The main reaction route for the three model compounds was the cleavage of the ether bonds to produce aromatic alkanes and phenol, and the latter was further reduced to cyclohexanol. Dioxasolv lignin depolymerization results exhibit a significant Caryl–O decrease over the Ni nanoparticles supported on Al2O3 with iso-propanol as the hydrogen source through 2D-HSQC-NMR analysis, which confirmed the transfer hydrogenolysis conclusion in the model study. This work provides an economical and environmentally-friendly method for the selective cleavage of lignin and lignin model compounds into value-added chemicals.
中文翻译:

在非均相镍催化剂的催化下,无需外部氢即可选择性裂解木质素和木质素模型化合物†
木质素中C芳基-O键的选择性氢解是从生物质生产燃料和化学原料的关键策略。目前,氢解主要使用氢气进行,氢气易燃且不可持续或不经济。在此,报道了在木质素模型中芳基醚氢解以及在Al 2 O 3负载的镍纳米粒子上二氧杂溶剂木质素的外部无氢工艺。动力学研究表明,三种模型化合物的转移氢解活性按以下顺序降低:苄基苯基醚(α-O-4)、2-苯基乙基苯基醚(β-O-4)和二苯基醚(4-O- 5),其与它们的结合能和活化能线性对应。三种模型化合物的主要反应路线是醚键断裂生成芳香烷烃和苯酚,后者进一步还原为环己醇。通过2D-HSQC-NMR分析,以异丙醇为氢源的Al 2 O 3负载的Ni纳米颗粒相比,二恶沙溶剂木质素解聚结果显示C芳基-O显着减少,证实了模型研究中的转移氢解结论。这项工作为木质素和木质素模型化合物选择性裂解为增值化学品提供了一种经济且环保的方法。
更新日期:2019-03-21
中文翻译:

在非均相镍催化剂的催化下,无需外部氢即可选择性裂解木质素和木质素模型化合物†
木质素中C芳基-O键的选择性氢解是从生物质生产燃料和化学原料的关键策略。目前,氢解主要使用氢气进行,氢气易燃且不可持续或不经济。在此,报道了在木质素模型中芳基醚氢解以及在Al 2 O 3负载的镍纳米粒子上二氧杂溶剂木质素的外部无氢工艺。动力学研究表明,三种模型化合物的转移氢解活性按以下顺序降低:苄基苯基醚(α-O-4)、2-苯基乙基苯基醚(β-O-4)和二苯基醚(4-O- 5),其与它们的结合能和活化能线性对应。三种模型化合物的主要反应路线是醚键断裂生成芳香烷烃和苯酚,后者进一步还原为环己醇。通过2D-HSQC-NMR分析,以异丙醇为氢源的Al 2 O 3负载的Ni纳米颗粒相比,二恶沙溶剂木质素解聚结果显示C芳基-O显着减少,证实了模型研究中的转移氢解结论。这项工作为木质素和木质素模型化合物选择性裂解为增值化学品提供了一种经济且环保的方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号