Environmental Pollution ( IF 7.6 ) Pub Date : 2019-03-20 , DOI: 10.1016/j.envpol.2019.03.065 Huifen Yang , Zhaofeng Li , Peng Fu , Ge Zhang
|
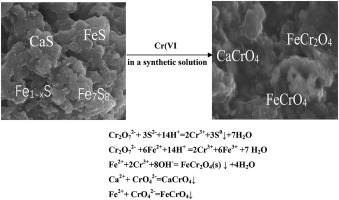
A novel carbonaceous material (NCM), prepared by the pyrolyzation of the oily sludge of tank bottom, was proposed to remove Cr(VI) from a synthetic solution for the first time. The effects of initial Cr(VI) concentration, NCM dosage and initial solution pH on Cr(VI) removal and the adsorption kinetics, the adsorption isothem were investigated. The removal mechanism was studied by comparing the surface properties of NCM before and after the Cr(VI) removal. The results showed that NCM can effectively remove Cr(Ⅵ) from the synthetic solution with the increase of solution pH at equilibrium. At the initial Cr(Ⅵ) concentrations of 40, 100, 150 and 250 mg/L and NCM dosages of 1, 3, 6 and 8 g/L with initial solution pH of 2, the removal efficiency of Cr(VI) was 95.5, 96.8, 95.2 and 81.2%, and the solution pH at equilibrium reached 2.3, 3.5, 5.8 and 7.5, respectively. NCM was suitable for Cr(Ⅵ) removal while the initial Cr(VI) concentration was less than 100 mg/L and initial solution pH was lower than 2.5. Most of Cr(VI) was removed by the reduction of Fe2+ and S2− in NCM to Cr(III) and with the generation of stable FeCr2O4. Some Cr(VI) may be removed by reacting with Fe2+ and Ca2+ to produce CaCrO4 and FeCrO4 on the NCM surface. The dissolution of CaAl2Si2O8 and CaS in the solution increased the solution pH at equilibrium. NCM has been proved to be a material with dual functions both chemical reduction and adsorption.
中文翻译:

使用罐底含油污泥制备的新型碳质材料从合成溶液中去除六价铬
提出了一种通过罐底含油污泥的热解制备的新型含碳材料(NCM),首次从合成溶液中去除Cr(VI)。研究了初始Cr(VI)浓度,NCM用量和初始溶液pH对Cr(VI)的去除以及吸附动力学,吸附等温线的影响。通过比较去除Cr(VI)前后NCM的表面性能,研究了去除机理。结果表明,在平衡状态下,随着溶液pH值的升高,NCM可以有效地从合成溶液中去除Cr(Ⅵ)。在初始Cr(Ⅵ)浓度为40、100、150和250 mg / L且NCM剂量为1、3、6和8 g / L且初始溶液pH为2时,Cr(VI)的去除效率为95.5分别为96.8、95.2和81.2%,并且溶液的pH值在平衡状态下分别达到2.3、3.5、5.8和7.5,分别。NCM适合去除Cr(Ⅵ),而初始Cr(VI)浓度小于100 mg / L,初始溶液pH小于2.5。通过还原铁去除了大部分Cr(VI)NCM中的2+和S 2−生成Cr(III),并生成稳定的FeCr 2 O 4。某些Cr(VI)可以通过与Fe 2+和Ca 2+反应以在NCM表面上生成CaCrO 4和FeCrO 4的方式除去。CaAl 2 Si 2 O 8和CaS在溶液中的溶解增加了溶液在平衡时的pH。NCM已被证明是具有化学还原和吸附双重功能的材料。































 京公网安备 11010802027423号
京公网安备 11010802027423号