当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cyclo-oligomerization of 6,12-Diethynyl Indeno[1,2-b]fluorenes via Diradical Intermediates
Organic Letters ( IF 4.9 ) Pub Date : 2015-11-02 00:00:00 , DOI: 10.1021/acs.orglett.5b03000 Xiangyu Fu 1 , Dahui Zhao 1
Organic Letters ( IF 4.9 ) Pub Date : 2015-11-02 00:00:00 , DOI: 10.1021/acs.orglett.5b03000 Xiangyu Fu 1 , Dahui Zhao 1
Affiliation
|
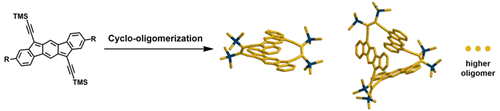
Indeno[1,2-b]fluorene derivatives with trimethylsilylethynyl substituents at the 6- and 12-positions were found to undergo cyclo-dimerization, cyclo-trimerization, and higher oligomerizations at room temperature. The cyclic dimer features a novel double-decker motif, composed of two face-to-face stacked bis(propadienylide)dihydroindeno[1,2-b]fluorenes with a short centroid-to-centroid distance of 3.50 Å. The existence of a cyclic trimer and higher oligomers was confirmed by mass spectroscopy and gel permeation chromatography. The results clearly demonstrate the diradical feature of the indeno[1,2-b]fluorene moiety.
中文翻译:

经由双自由基中间体的6,12-二乙炔基茚并[1,2- b ]芴环低聚
发现在6-位和12-位具有三甲基甲硅烷基乙炔基取代基的茚并[1,2- b ]芴衍生物在室温下进行环二聚,环三聚和更高的低聚。环状二聚体的特征是新颖的双层图案,由两个面对面堆叠的双(丙二烯基)二氢茚并[1,2- b ]芴构成,它们的质心间距离短,为3.50Å。质谱和凝胶渗透色谱法证实了环状三聚体和高级低聚物的存在。结果清楚地证明了茚并[1,2- b ]芴部分的双自由基特征。
更新日期:2015-11-02
中文翻译:

经由双自由基中间体的6,12-二乙炔基茚并[1,2- b ]芴环低聚
发现在6-位和12-位具有三甲基甲硅烷基乙炔基取代基的茚并[1,2- b ]芴衍生物在室温下进行环二聚,环三聚和更高的低聚。环状二聚体的特征是新颖的双层图案,由两个面对面堆叠的双(丙二烯基)二氢茚并[1,2- b ]芴构成,它们的质心间距离短,为3.50Å。质谱和凝胶渗透色谱法证实了环状三聚体和高级低聚物的存在。结果清楚地证明了茚并[1,2- b ]芴部分的双自由基特征。































 京公网安备 11010802027423号
京公网安备 11010802027423号