当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis, fungicidal activity and molecular docking studies of novel 2-((2-hydroxyphenyl)methylamino)acetamide derivatives.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-03-20 , DOI: 10.1016/j.bmc.2019.03.040 Zilong Tang 1 , Xinxing Li 2 , Yuan Yao 2 , Yongcun Qi 2 , Ming Wang 2 , Ningning Dai 2 , Yuhao Wen 2 , Yichao Wan 3 , Lifen Peng 3
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-03-20 , DOI: 10.1016/j.bmc.2019.03.040 Zilong Tang 1 , Xinxing Li 2 , Yuan Yao 2 , Yongcun Qi 2 , Ming Wang 2 , Ningning Dai 2 , Yuhao Wen 2 , Yichao Wan 3 , Lifen Peng 3
Affiliation
|
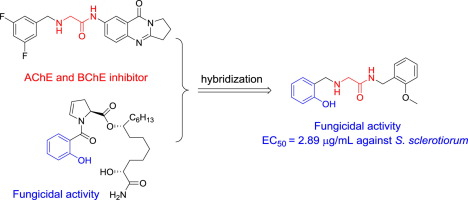
A series of novel 2-hydroxyphenyl substituted aminoacetamides was designed by molecular hybridization of the aminoacetamide scaffold and 2-hydroxyphenyl motif. The target compounds were synthesized and their fungicidal activities were evaluated. Some of the target compounds showed excellent antifungal activities against S. sclerotiorum and P. capsici. Significantly, compounds 5e displayed the most potent activity against S. sclerotiorum with EC50 = 2.89 µg/mL, which was lower than that of commercial chlorothalonil. The systematic studies provided strong confidence that the hydroxyl group and the carbonyl group are crucial for the fungicidal activity. Molecular docking studies suggest that SDH enzyme could be one of the potential action targets of our compounds.
中文翻译:

新的2-((2-羟苯基)甲基氨基)乙酰胺衍生物的设计,合成,杀真菌活性和分子对接研究。
通过氨基乙酰胺支架和2-羟基苯基基序的分子杂交,设计了一系列新颖的2-羟基苯基取代的氨基乙酰胺。合成了目标化合物并评估了其杀真菌活性。一些目标化合物对S. sclerotiorum和辣椒辣椒具有极好的抗真菌活性。值得注意的是,化合物5e表现出最强的抵抗链球菌的活性,EC50 = 2.89 µg / mL,低于商品百菌清的活性。系统研究提供了强大的信心,即羟基和羰基对于杀真菌活性至关重要。分子对接研究表明,SDH酶可能是我们化合物潜在的作用靶标之一。
更新日期:2019-03-20
中文翻译:

新的2-((2-羟苯基)甲基氨基)乙酰胺衍生物的设计,合成,杀真菌活性和分子对接研究。
通过氨基乙酰胺支架和2-羟基苯基基序的分子杂交,设计了一系列新颖的2-羟基苯基取代的氨基乙酰胺。合成了目标化合物并评估了其杀真菌活性。一些目标化合物对S. sclerotiorum和辣椒辣椒具有极好的抗真菌活性。值得注意的是,化合物5e表现出最强的抵抗链球菌的活性,EC50 = 2.89 µg / mL,低于商品百菌清的活性。系统研究提供了强大的信心,即羟基和羰基对于杀真菌活性至关重要。分子对接研究表明,SDH酶可能是我们化合物潜在的作用靶标之一。







































 京公网安备 11010802027423号
京公网安备 11010802027423号