Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-02-10 , DOI: 10.1016/j.bmc.2018.02.005 Takashi Nakahata , Kazuyuki Tokumaru , Yoshiteru Ito , Naoki Ishii , Masaki Setoh , Yuji Shimizu , Toshiya Harasawa , Kazunobu Aoyama , Teruki Hamada , Masakuni Kori , Kazuyoshi Aso
|
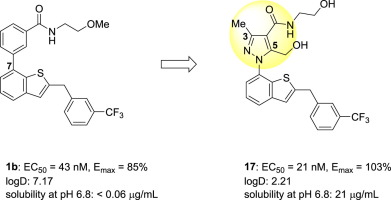
G-protein-coupled receptor 52 (GPR52) is classified as an orphan Gs-coupled G-protein-coupled receptor. GPR52 cancels dopamine D2 receptor signaling and activates dopamine D1/N-methyl-d-aspartate receptors via intracellular cAMP accumulation. Therefore, GPR52 agonists are expected to alleviate symptoms of psychotic disorders. A novel series of 1-(benzothiophen-7-yl)-1H-pyrazole as GPR52 agonists was designed and synthesized based on compound 1b. Compound 1b has been reported by our group as the first orally active GPR52 agonist, but high lipophilicity and poor aqueous solubility still remained as issues for candidate selection. To resolve these issues, replacement of the benzene ring at the 7-positon of compound 1b with heterocylic rings, such as pyrazole and pyridine, was greatly expected to reduce lipophilicity to levels for which calculated logD values were lower than that of compound 1b. While evaluating the pyrazole derivatives, introduction of a methyl substituent at the 3-position of the pyrazole ring led to increased GPR52 agonistic activity. Moreover, additional methyl substituent at the 5-position of the pyrazole and further introduction of hydroxy group to lower logD led to significant improvement of solubility while maintaining the activity. As a result, we identified 3-methyl-5-hydroxymethyl-1H-pyrazole derivative 17 (GPR52 EC50 = 21 nM, Emax = 103%, logD = 2.21, Solubility at pH 6.8 = 21 μg/mL) with potent GPR52 agonistic activity and good solubility compared to compound 1b. Furthermore, this compound 17 dose-dependently suppressed methamphetamine-induced hyperlocomotion in mice.
中文翻译:

设计和合成1-(1-苯并噻吩-7-基)-1 H-吡唑,一种新型的G蛋白偶联受体52(GPR52)激动剂
G蛋白偶联受体52(GPR52)被归类为孤儿Gs偶联G蛋白偶联受体。GPR52取消多巴胺D2受体信号传导和激活多巴胺D1 / Ñ甲基d经由细胞内cAMP积累天冬氨酸受体。因此,预期GPR52激动剂可减轻精神病的症状。基于化合物1b,设计合成了一系列新型的1-(苯并噻吩-7-基)-1H-吡唑作为GPR52激动剂。化合物1b我们的研究小组已将其报告为第一种口服活性GPR52激动剂,但高亲脂性和较差的水溶性仍然是候选药物选择的问题。为了解决这些问题,非常希望用杂环如吡唑和吡啶取代化合物1b的7位上的苯环,从而将亲脂性降低至计算出的logD值低于化合物1b的水平。在评估吡唑衍生物时,在吡唑环的3-位引入甲基取代基导致增加的GPR52激动活性。此外,在吡唑的5-位的另外的甲基取代基和进一步引入羟基以降低logD导致在保持活性的同时溶解度显着提高。结果,我们确定了有效的3-甲基-5-羟甲基-1 H-吡唑衍生物17(GPR52 EC 50 = 21 nM,E max = 103%,logD = 2.21,pH 6.8下的溶解度= 21μg/ mL)与化合物1b相比,GPR52具有激动活性和良好的溶解性。此外,该化合物17 剂量依赖性抑制甲基苯丙胺诱发的小鼠运动过度。






























 京公网安备 11010802027423号
京公网安备 11010802027423号