当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Surface Tailoring Oxygen Functional Groups on Carbon Spheres for Capacitive Mechanistic Study
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-03-19 00:00:00 , DOI: 10.1021/acsami.8b22370 Dongdong Zhang 1, 2 , Jianlong Wang 1, 2 , Chong He 2 , Yuzi Wang 1, 2 , Taotao Guan 1, 2 , Jianghong Zhao 3 , Jinli Qiao 4 , Kaixi Li 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-03-19 00:00:00 , DOI: 10.1021/acsami.8b22370 Dongdong Zhang 1, 2 , Jianlong Wang 1, 2 , Chong He 2 , Yuzi Wang 1, 2 , Taotao Guan 1, 2 , Jianghong Zhao 3 , Jinli Qiao 4 , Kaixi Li 1, 2
Affiliation
|
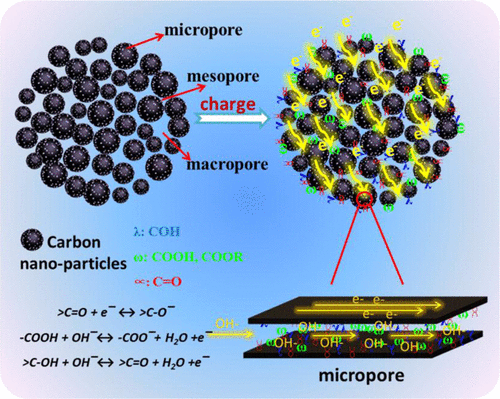
Porous carbons represent a typical class of electrode materials for electric double-layer capacitors. However, less attention has been focused on the study of the capacitive mechanism of electrochemically active surface oxygen groups rooted in porous carbons. Herein, the degree and variety of oxygen surface groups of HNO3-modified samples (N-CS) are finely tailored by a mild hydrothermal oxidation (0.0–3.0 mol L–1), while the micro–meso–macroporous structures are efficiently preserved from the original sample. Thus, N-CS is a suitable carrier for separately discussing the contribution of oxygen functional groups to the electrochemical property. The optimized N-CS shows a high capacitance of 279.4 F g–1 at 1 A g–1, exceeding 52.8% of pristine carbon sphere (CS) (182.8 F g–1 at 1 A g–1) in KOH electrolyte. On further deconvoluting the redox peaks of cyclic voltammetry curves, we find that the pseudocapacitance not only associates with the surface-controlled faradic reaction at high scan rate but also dramatically stems from the diffusion-controlled capacitance through potassium and hydroxyl ion insertion/deinsertion into the underutilized micropores at low scan rate. The assembled supercapacitor based on N-CS presents a stable energy density of 5 Wh kg–1 over a wide range of power density of 250–5000 W kg–1, which is higher than 0.0N-CS in KOH electrolyte. In TEABF4 electrolyte, the N-CS supercapacitor has an energy density of 26.9 Wh kg–1 at the power density of 1350 W kg–1 and exhibits excellent cycling stability with a capacitance retention of 93.2% at 2 A g–1 after 10 000 cycles. These results demonstrate that surface oxygen groups alter the capacitive mechanism and contribution of porous carbons.
中文翻译:

用于电容机理的碳球上合理的表面定制氧官能团
多孔碳代表用于双电层电容器的一类典型的电极材料。然而,较少关注集中在对源自多孔碳中的电化学活性表面氧基团的电容机理的研究。在此,HNO 3改性样品(N-CS)的氧表面基团的程度和种类通过温和的水热氧化(0.0–3.0 mol L –1)进行了精确调整,而微介孔结构则得到了有效保护来自原始样本。因此,N-CS是用于分开讨论氧官能团对电化学性能的贡献的合适载体。经过优化的N-CS在1 A g –1时显示出279.4 F g –1的高电容,超出原始碳球(CS)的52.8%(182.8 F G -1 1 A G -1)的KOH电解质。在进一步解卷积循环伏安曲线的氧化还原峰时,我们发现假电容不仅与高扫描速率下的表面控制法拉第反应有关,而且还显着源自通过钾和氢氧根离子插入/反插入到电极中的扩散控制电容。低扫描率的未充分利用的微孔。基于N-CS的组装超级电容器在250-5000 W kg -1的大功率密度范围内表现出5 Wh kg –1的稳定能量密度,高于KOH电解质中的0.0N-CS。在TEABF 4中作为电解质,N-CS超级电容器在1350 W kg –1的功率密度下具有26.9 Wh kg –1的能量密度,并具有出色的循环稳定性,在1万次循环后,在2 A g –1下的电容保持率为93.2%。这些结果表明表面氧基团改变了多孔碳的电容机制和贡献。
更新日期:2019-03-19
中文翻译:

用于电容机理的碳球上合理的表面定制氧官能团
多孔碳代表用于双电层电容器的一类典型的电极材料。然而,较少关注集中在对源自多孔碳中的电化学活性表面氧基团的电容机理的研究。在此,HNO 3改性样品(N-CS)的氧表面基团的程度和种类通过温和的水热氧化(0.0–3.0 mol L –1)进行了精确调整,而微介孔结构则得到了有效保护来自原始样本。因此,N-CS是用于分开讨论氧官能团对电化学性能的贡献的合适载体。经过优化的N-CS在1 A g –1时显示出279.4 F g –1的高电容,超出原始碳球(CS)的52.8%(182.8 F G -1 1 A G -1)的KOH电解质。在进一步解卷积循环伏安曲线的氧化还原峰时,我们发现假电容不仅与高扫描速率下的表面控制法拉第反应有关,而且还显着源自通过钾和氢氧根离子插入/反插入到电极中的扩散控制电容。低扫描率的未充分利用的微孔。基于N-CS的组装超级电容器在250-5000 W kg -1的大功率密度范围内表现出5 Wh kg –1的稳定能量密度,高于KOH电解质中的0.0N-CS。在TEABF 4中作为电解质,N-CS超级电容器在1350 W kg –1的功率密度下具有26.9 Wh kg –1的能量密度,并具有出色的循环稳定性,在1万次循环后,在2 A g –1下的电容保持率为93.2%。这些结果表明表面氧基团改变了多孔碳的电容机制和贡献。















































 京公网安备 11010802027423号
京公网安备 11010802027423号