当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Cyclohexadienone Containing Spiroketals via DyKat Ketalization/oxa-Michael Addition Cascade
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-03-18 00:00:00 , DOI: 10.1021/acs.joc.9b00371 Reddy Rajasekhar Reddy 1 , Shibaram Panda 1 , Prasanta Ghorai 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-03-18 00:00:00 , DOI: 10.1021/acs.joc.9b00371 Reddy Rajasekhar Reddy 1 , Shibaram Panda 1 , Prasanta Ghorai 1
Affiliation
|
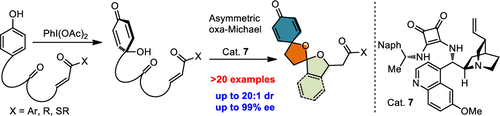
An oxidative dearomatization of phenol followed by a dynamic kinetic (DyKat) ketalization/oxa-Michael addition cascade using cinchona alkaloid-based chiral bifunctional amino-squaramide catalysts is reported. A broad array of sterically hindered [5,5]-spiroketals attached to a cyclohexadienone moiety in spiro-fashion is synthesized in an enantiopure form. Further, the methodology was optimized and extended to the corresponding benzannulated [5,5]-spiroketals attached to a cyclohexadienone moiety in spiro-fashion. In general, good yields and excellent diastereoselectivies and enantioselectivities (up to 20:1 dr and up to 99% ee) were obtained.
中文翻译:

DyKat缩酮化/ oxa-Michael加成级联反应合成含螺缩酮的环己二酮
报道了使用金鸡纳生物碱基手性双官能氨基-方酸酰胺催化剂对苯酚进行氧化脱芳香化反应,然后进行动态动力学(DyKat)缩酮化/ oxa-Michael加成级联反应。以对映体纯净的形式合成了各种以螺旋形式连接到环己二烯酮部分的位阻[5,5]螺酮。此外,该方法进行了优化,并扩展到螺旋时尚中连接到环己二烯酮部分的相应苯并[5,5]-螺酮。通常,获得了良好的收率和优异的非对映选择性和对映选择性(高达20:1 dr和高达99%ee)。
更新日期:2019-03-18
中文翻译:

DyKat缩酮化/ oxa-Michael加成级联反应合成含螺缩酮的环己二酮
报道了使用金鸡纳生物碱基手性双官能氨基-方酸酰胺催化剂对苯酚进行氧化脱芳香化反应,然后进行动态动力学(DyKat)缩酮化/ oxa-Michael加成级联反应。以对映体纯净的形式合成了各种以螺旋形式连接到环己二烯酮部分的位阻[5,5]螺酮。此外,该方法进行了优化,并扩展到螺旋时尚中连接到环己二烯酮部分的相应苯并[5,5]-螺酮。通常,获得了良好的收率和优异的非对映选择性和对映选择性(高达20:1 dr和高达99%ee)。































 京公网安备 11010802027423号
京公网安备 11010802027423号